Beyond NPV: Quantifying Patient Benefit in Cell & Gene Therapies
Picture an investment committee reviewing two first-in-class gene-editing programs. Financial models show identical risk-adjusted NPVs, yet one promises four times the projected patient benefit. Which wins the vote? The story isn’t about any single foundation; it’s about a recurring dilemma in CGT financing where dollars and patient impact don’t always point to the same deal.
Traditional rNPV remains the lingua franca of venture finance, but CGT’s high R&D costs, small patient pools and curative ambitions make monetary returns unusually volatile. Layering patient impact alongside NPV offers a stabilising compass, ensuring capital flows toward programmes that move both the balance-sheet and the survival curve. Just last week, Philip Brainin (Associate at Sound Bioventures) published a new framework in Nature Biotechnology to measure the societal impact of venture investments in life sciences.
Quick Primer on SALYs & SADYs
Brainin’s framework proposes two patient-centric metrics tailored for investors. Science-Adjusted Life Years (SALYs) and Science-Adjusted Disability Years (SADYs) building on global health metrics like DALYs and QALYs but adjust for:
Probability of Scientific Success: SALYs are discounted by the likelihood a therapeutic candidate clears regulatory hurdles.
Equity Ownership: SALYs can be normalized per $ invested, per share class, or per co-investor share creating comparability across venture and public-private deals.
Where DALYs account for disease burden lost, SALYs forecast life-years gained a new measure grounded in both biology and market realism. SALYs and SADYs incorporate four key elements: (i) disease incidence, (ii) target-product-profile reach, (iii) change in likelihood-of-approval, and (iv) survival or disability benefit into a single number that discounts for scientific risk while scaling for real epidemiology.
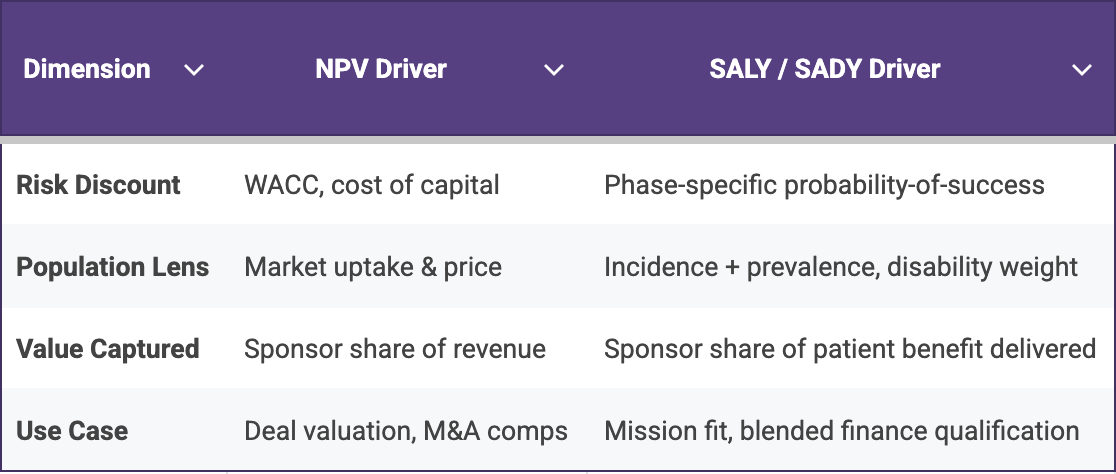
NPV reaches first for finance levers: discount rates, price and market uptake, while the SALY/SADY construct substitutes phase-specific probabilities of success, incidence and disability weights. The side-by-side comparison in Table 1 below makes the contrast explicit and underscores why the two metrics work best together: NPV flags capital efficiency, while SALY/SADY illuminates the magnitude of patient impact.
Table 1. Financial levers dominate NPV calculations, whereas disease burden and probability estimates dominate SALY/SADY making the two metrics complementary rather than redundant.
Cell and Gene Therapies: The Ultimate Stress-Test
Because single-dose curative therapies concentrate an entire lifetime of revenue into an upfront payment, traditional rNPV estimates can fluctuate sharply with every adjustment to price assumptions. In contrast, SALYs ground the analysis in quantified patient benefit, providing a more stable indicator of programme value. For example, a one-time prime-editing treatment targeting just 400 patients may still generate several thousand discounted SALYs, demonstrating substantial societal impact even when the commercial total addressable market remains modest. That analysis may be crucial for foundations deciding between grants and equity, as well as HTA agencies assessing early economic value and impact funds seeking to prove additionality.
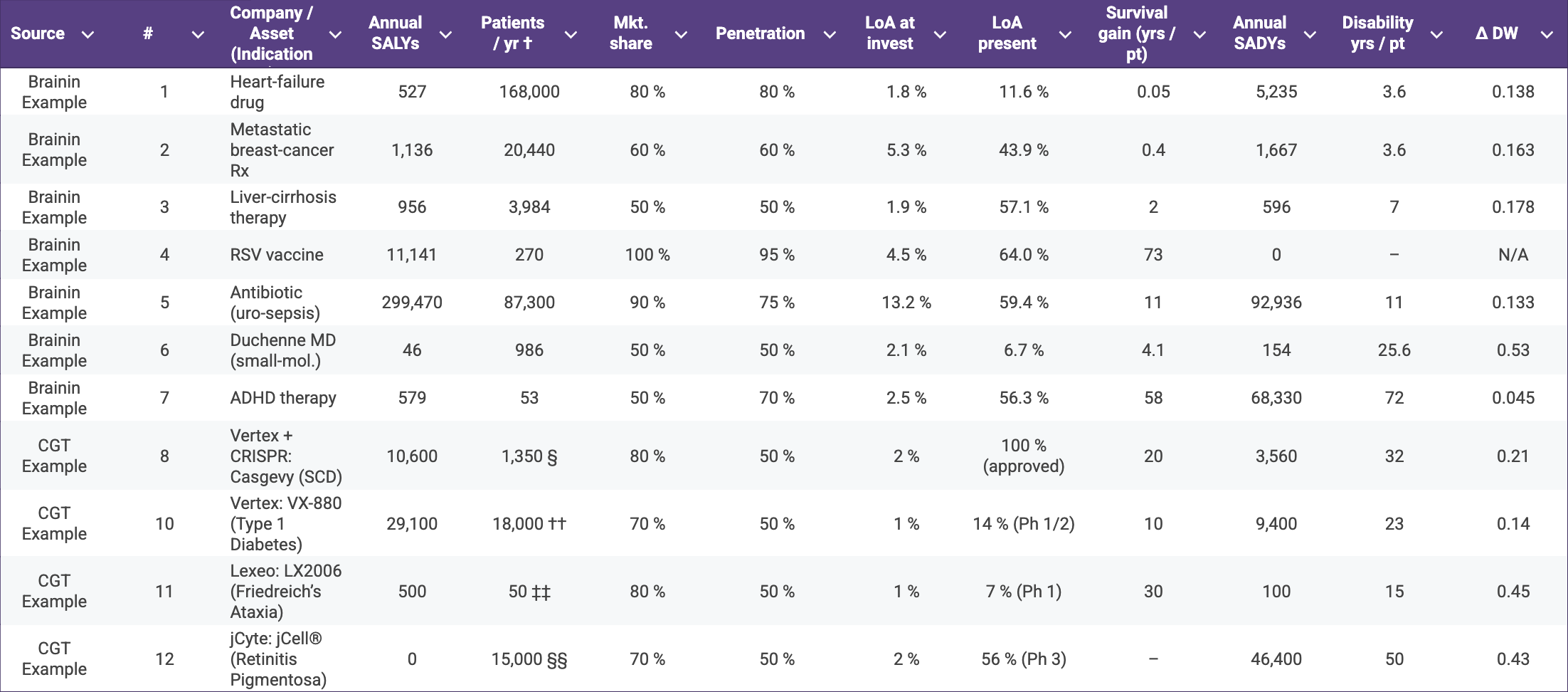
The matrix below illustrates how SALY and SADY calculations can re-rank programmes once patient-benefit intensity is considered. High-incidence mainstream drugs (e.g., an antibiotic for urosepsis) unsurprisingly dominate absolute SALY totals, yet several CGT examples; Casgevy for sickle-cell disease, Vertex’s VX-880 for type 1 diabetes, and Lexeo’s LX2006 for Friedreich’s ataxia deliver double- and triple-digit SALY counts from comparatively smaller patient pools.
Table 2. Annual Science-Adjusted Life-Years (SALYs) and Science-Adjusted Disability-Years (SADYs) for representative therapeutics spanning Brainin’s original examples and a selection of cell- and gene-therapy (CGT) assets†
† Incident U.S. patient population, mirroring Brainin’s convention.
§ Birth prevalence ≈ 1 : 2,024 (CDC) → ~1,830 births / yr; halving to those with ≥ 2 VOCs ⇒ 1,350.
¶ U.S. annual DMD diagnoses estimated at 300–400 boys / yr (1 : 5 000 male births).
†† CDC reports just over 18,000 youth diagnosed with T1D each year.
‡‡ FA affects 1/50,000 people; 4 M births × 1/50,000 ⇒ ~80 births; 60 % meet cardiac-gene-therapy criteria ⇒ ~50.
§§ GenSight estimates 15,000–20,000 new RP vision-loss cases per year in the US + EU; mid-point used for US.
Value for Patient Alliances & Impact Investors
The comparison highlights why impact-minded investors, foundations, and HTA agencies should weigh both population size and per-patient life-year gain rather than relying on rNPV alone: a modest-TAM curative can still generate a disproportionate share of risk-adjusted patient benefit. Moreover, quantified patient benefit can arm advocates with data for accelerated review petitions, outcome-based pricing arguments and R&D tax-credit campaigns.
Inserting SALY forecasts into horizon-scans lets bodies like NICE or FDA’s Health-Equity initiative flag high-impact indications earlier. Likewise, manufacturing grants, fee waivers or voucher tweaks can be recalibrated to favour high-SALY but commercially challenging diseases.
Implementation Playbook
Lonrú’s VantagePoint™ Suite can combine global burden of disease epidemiology with our proprietary phase-transition probability library to generate turnkey SALY/SADY dashboards. Beyond the analytics, Lonrú can partner directly with patient alliances to run evidence-gathering workshops, analyse and validate disability weights, and co-author advocacy briefs that translate SALY insights into concrete asks for regulators and payers. Lonrú Consulting operates as a neutral translator between financial, clinical and patient voices. By integrating Brainin’s novel SALY/SADY calculus into our advisory workflows, we can help stakeholders steer capital and policy toward truly transformative CGT programmes.