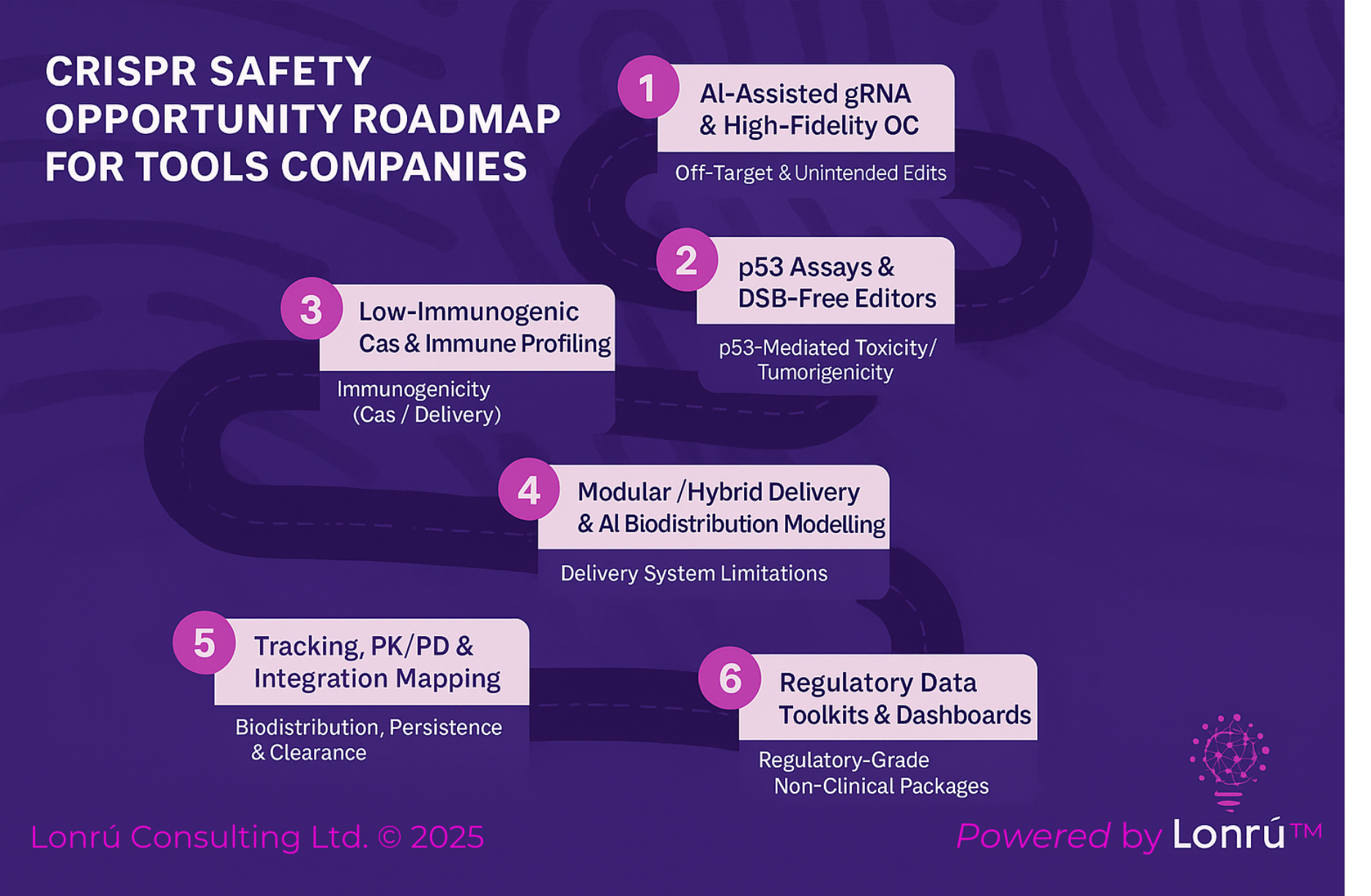
Beyond the Repository: Transforming Contracts into Active Risk Intelligence
The Statistic That Keeps CFOs Awake
If you asked your General Counsel where your contracts are, they would point to a secure folder. But if your CFO asked, "What is our total un-budgeted exposure if inflation hits 5% next quarter?" - the room would go silent.
For most Biopharma companies, contracts are Dead Text. They are static PDFs stored in a repository, only reviewed when a dispute arises.
This static approach is expensive. According to research from World Commerce & Contracting (WorldCC), the average organization loses 9.2% of its annual revenue to contract value leakage.
This money doesn't disappear in a heist; it leaks out slowly through missed milestones, unwanted auto-renewals, and untracked obligation clauses that never get enforced.
The Big Tech Trap
The typical industry response is to buy a robust Contract Lifecycle Management (CLM) platform. It sounds like the right move, yet Gartner predicts that nearly 50% of first-time CLM implementations fail to deliver their expected value.
Why? Because most CLMs are designed for Storage, not Intelligence. They are excellent digital filing cabinets, but they are terrible at answering financial questions.
The Solution: The Portfolio Risk Engine
At Lonrú Studios™, we believe your contract portfolio shouldn't be a library; it should be a Living Ledger.
We built a model Portfolio Risk Engine to demonstrate the difference between storing data and interrogating it. Instead of a list of files, it gives executives a reactive dashboard of Aggregate Risk.
See the Executive Dashboard below:
Feature Highlight: The Inflation Stress Test
In the bottom-left of the dashboard above, you’ll see the Inflation Scenario module.
The Problem: Many vendor MSAs contain CPI-Linker clauses that allow prices to rise automatically with inflation. In a static CLM, these clauses are buried on a page of a PDF.
The Engine's Solution: We built a Scenario Toggle. When the CFO flips the switch to "Simulate 5% Inflation Spike," the engine instantly sums the value of only the contracts with those specific clauses.
The Result: A theoretical legal clause becomes a hard financial number (e.g., $145,000 in un-budgeted exposure) in 3 seconds.
Feature Highlight: The Exposure Matrix
The Exposure Matrix (top left) replaces the yearly audit with a live heatmap.
X-Axis: Financial Value ($).
Y-Axis: Liability Cap Multiplier (1x, 2x, Unlimited).
The Insight: It instantly isolates the "Kill Zone"—low-value vendors that carry Unlimited Liability (the pink dots on the far left). These are the silent risks that generic tools often miss.
From Repository to Radar
The difference between a "Repository" and a "Risk Engine" is architecture. By structuring your contracts with specific metadata tags - CPI_Linker, Liability_Cap, Notice_Period - you turn legal text into business intelligence.
Stop managing million-dollar liabilities in static folders. Let’s architect a Risk Engine that protects your bottom line.
The High Cost of Disconnected Workflows: Why Ad-Hoc Alignment Is Bleeding Revenue
Last week, in Operational Latency: Solving the Structural Gap Between Strategy and Execution, we defined the "silent killer" of biotech efficiency: the lag time between a strategic decision and its operational implementation. Today, we are looking at where that latency costs the most money: Strategic Alliances.
The 9% Leakage Problem
As the industry rushes to fill revenue gaps before the 2028 patent cliffs, the complexity of partnering deals has outpaced the infrastructure used to manage them.
The statistics are sobering. According to research from the Association of Strategic Alliance Professionals (ASAP), failure rates for alliances managed on an ad-hoc basis can reach as high as 80%. Even more critical for the CFO’s office is data from World Commerce & Contracting, which reveals that the average organization loses over 9% of annual revenue to Contract Value Leakage.
This leakage doesn't usually happen because the science failed. It happens because the Legal intent (the contract), the Operational reality (the alliance manager), and the Financial forecast (the spreadsheet) are disconnected.
Case Analysis: The $180 Million Misunderstanding
When these three functions operate in silos, the result is rarely just confusion - it is litigation.
Consider the dispute between Alexion Pharmaceuticals and Syntimmune. Following a $1.2B acquisition, a conflict arose regarding a $130M milestone payment. The core issue was a misalignment between the operational decision to pause a trial and the Commercially Reasonable Efforts clause in the merger agreement.
The court eventually ordered Alexion to pay over $180 million. This serves as a stark warning: when your Contract Logic and your Operational Triggers drift apart, the financial risk becomes material.
The Solution: Connected Intelligence
To stop this leakage, we must move beyond static spreadsheets and clunky software stitched together. We need Systemic Alignment - architecture that connects the legal obligation directly to the operational trigger.
We built the Alliance Radar model to demonstrate what this looks like in practice. It connects three distinct stakeholder views into a single, reactive system:
The Watchtower (Alliance View): Detects real-world signals (e.g., Clinical Data, Regulatory News), notifying the alliance manager of critical intel just-in-time.
The Forecast (Finance View): Instantly recalculates cash flow based on signals verified by the alliance manager.
The Library (Legal View): Maps every dollar back to the specific contract clause.
Explore the model below:
Interactive Model
In this simulation, you can act as the Alliance Manager. Verify a Press Release in the Watchtower tab and watch how the system automatically updates the Finance Forecast - removing the need for manual data entry and ensuring the Single Source of Truth remains accurate.
From Model to Architecture
The Alliance Radar is a simplified public model, and while it addresses a universal problem the functional solution is unique to each pipeline.
At Lonrú Studios™, we do not believe in one-size-fits-all platforms. We operate as an architecture studio, building bespoke micro-tools tailored to your Master Service Agreements and your internal governance. We help you architect the connection between Legal, Finance, and Operations - closing the gap where that 9% of revenue likes to hide.
Stop managing million-dollar assets in disconnected spreadsheets. Let’s architect a solution that fits.
Operational Latency: Solving the Structural Gap Between Strategy and Execution
The Diagnosis: The Two Extremes of Data Hell
In 2025, I met with a Life Sciences executive who was frustrated by data visibility across their team. In the year prior, they had completed a six-figure investment in a centralized enterprise BI implementation (think Tableau, PowerBI).
"We bought the Ferrari," they said. "But my team is still walking."
When we audited their workflow, we found the structural reality. The team wasn't using the expensive dashboards. They were exporting the data to CSV, opening it in Excel, and doing their actual work there.
Why? Because organizations are currently trapped between two broken extremes:
The "Shadow IT" (Excel/Spreadsheets):
Pros: Infinite flexibility. The user is in control.
Cons: Fragile, version-control nightmares, insecure, and manual. It is a house of cards waiting to collapse.
The "Golden Cage" (Enterprise BI):
Pros: Secure, compliant, and visually polished.
Cons: Rigid, read-only, and generic. You can look at the data, but you can’t act on it. If you need a new column, you have to file a ticket with IT and wait six weeks.
The opportunity for 2026 teams lies in solving this Operational Latency.
The Solution: The Case for Micro-Tools
Strategy fails when tools don't fit the hand that wields them. Through Lonrú Studios™, we avoid force-feeding All-in-One platforms to specialized teams. Instead, we architect Micro-Tools. The value proposition for the modern enterprise is simple: Speed, Specificity, and Ownership.
In the era of modern architecture, you no longer need to wait 12 months for an IT roadmap or pay heavy license fees for bloated software where your team uses only 5% of the features. Micro-tools allow your team to deploy a targeted solution in weeks, ensuring that your budget goes towards functionality, not seat licenses. Most importantly, because the tool is built around your exact workflow, user adoption becomes automatic rather than enforced.
To illustrate it, we are sharing a functional model of a Clinical Command Center. We designed it to solve a classic friction point: The disconnect between Clinical Operations (who need speed) and Finance (who need control).
See it in action below:
How It Works: This isn't two disconnected spreadsheets. It is one single data core viewed through two specific lenses:
On the Left (The Ops View): A drag-and-drop Kanban board. The Ops team simply moves a site from Contracting to Active.
On the Right (The Finance View): A reactive ledger. Watch the Projected Liability metric. As soon as Ops moves that card, the liability drops and an Action Required invoice appears instantly for Finance.
The ROI: Why This Wins
By building the bridge specifically for this workflow, we unlock efficiencies that neither Excel nor Tableau can offer:
One Source of Truth: No more email chains asking "Is this site active yet?" The Operations view and the Finance view remain in perfect, real-time sync.
Total Reactivity: Information flows instantly. If a site activates at 9:00 AM, Finance can release the grant at 9:01 AM.
Auditable Logs: Unlike Excel, every drag-and-drop action is timestamped. You get the flexibility of a spreadsheet with the compliance of a bank.
Tailored Views: Ops gets the agility of a Kanban board; Finance gets the rigidity of a ledger. Both teams get exactly what they need to do their job.
The Insight: Build the Scalpel, Not the Swiss Army Knife
In our work with Lonrú Studios™ and VantagePoint™, we’ve learned that specificity wins.
Ultimately, this is a question of value capture. When you subscribe to a generic platform, you are renting a capability that your competitors also have. When you architect a micro-tool, you are building a proprietary asset. The most valuable companies of 2026 won't be the ones with the most expensive software licenses; they will be the ones that own their own operational intelligence.
Don’t force your team to choose between chaos (Excel) and rigidity (Enterprise BI). Let’s build the tool they actually need.
Escaping AI Pilot Purgatory: Why 2025 Was the Year of the Builder
The Diagnosis: The ROI Paradox
If you look at the macro-level data from 2025, the narrative is confusing. Reports from Deloitte and McKinsey confirm that enterprise AI investment hit record highs - 85-90% of organizations increased their spend. Yet, the same reports highlight a Return on Investment Paradox. Most organizations are stuck in AI Pilot Purgatory, trapped by data silos, rigid legacy workflows, and the inability to move from proof-of-concept to production.
At Lonrú, we observed a distinct "Innovation Gap." While large corporations struggled with enterprise-wide transformation, agile teams focused on "micro-productivity" began outpacing them.
Our hypothesis for 2025 was simple: Strategies fail because the internal tools to execute them don't exist. We didn't wait for digital transformation. We built the specific, functional tools required to fix the problem today.
The Solution: 100 Tools in 12 Months
We spent the year traveling to major innovation hubs across Ireland, the UK, Europe, and the USA - validating this hypothesis. The market didn't need more slide decks; it needed functional architecture.
In 2025, Lonrú Studios™ deployed over 100 interactive tools. We moved clients off static spreadsheets and into reactive SQL environments. The demand wasn't for AI magic, but for three specific pillars of utility:
Operational Excellence: Six Sigma dashboards to instantly visualize process bottlenecks.
Commercial Strategy: Interactive partnership mapping to replace static market reports.
Engagement: Investor portals that track sentiment rather than just open rates.
Lonrú Studios™: Validating the Depth Hypothesis
The most common objection we hear is that decision-makers have short attention spans. "Make it simple," we are told. "Give me the answer in 5 seconds."
Our data proves otherwise.
In 2025, our most popular public tool, the VantagePoint™ CRISPR Clinical Trial Dashboard, a reactive tool tracking the global landscape of interventional trials. In an era of doom-scrolling, our users - scientists, investors, and strategists spent an average of one hour actively exploring the global data landscape through their own interactive lens. This validates a core Lonrú principle: Actionable insight requires depth. Our users didn't want a summary; they wanted to explore the data, filter the variables, and answer complex questions themselves. We don’t build a reports; we build resources.
The Geography of Innovation
Where is this deep engagement coming from? Our analytics paint a clear picture of the global R&D landscape.
Our user base (China, UK, USA, Europe) reflects the nations that are actively decentralizing their R&D:
China: We see rapid adoption driven by government support for Digital Therapeutics (DTx) and quantum-enhanced ML pipelines.
United Kingdom: The UK maintains its status as a clinical research powerhouse, aided by the MHRA’s evolving digital infrastructure.
USA: Continues to lead via FDA safe harbor sandboxes that encourage AI in trial design.
Singapore & Germany: Emerging as hubs prioritizing "Sustainable Intelligent Ecosystems" over legacy systems.
The takeaway is binary: You are either an early adopter in these hubs, or you are falling behind.
2026: Stop Piloting, Start Building
The Fast Follower strategy is dead. The complexity of the biological and digital landscapes in 2026 demands more than just advice. It requires the technical capacity to execute.
At Lonrú, we bridge that gap. We provide the PhD and MBA-level strategy to understand the problem, and the Studio-level engineering to build the solution.
Do you need a strategy that works? Or the tools to build it? Let's talk.
Talking Turkey: How CGT-Enabling Technologies Could Strengthen Future Biosecurity
Seasonal Pressures, Shared Challenges
As Thanksgiving approaches in the United States, families prepare for the rituals that anchor the holiday. This year, however, the celebration carries a familiar unease. The US and other regions around the globe are navigating renewed concerns about avian influenza in poultry flocks, prompting closer surveillance and heightened uncertainty for producers. These outbreaks highlight how vulnerable even the most established food traditions can be when confronted with fast-moving viruses.
While avian influenza is commonly framed as an agricultural issue, its implications run far deeper. The virus evolves quickly, traverses borders, and increasingly tests the limits of traditional containment practices. To the general public, the impact may appear only in the form of fluctuating prices or occasional supply worries. To those observing the broader arc of biotechnology, these disruptions signal the growing importance of scientific tools that can deepen resilience across entire food systems.
The Expanding Influence of CGT-Enabling Technologies
An interesting and consequential link is emerging between the technologies that support cell and gene therapy development and the tools needed to strengthen agricultural biosecurity. Although these technologies were designed for human therapeutic innovation, their underlying capabilities are surprisingly well-suited to the challenges facing modern livestock systems.
Rapid genomic sequencing allows scientists to follow how avian influenza evolves, supporting earlier detection and more informed responses. Gene editing platforms, which have transformed research in human therapeutics, are opening new avenues to understand and potentially influence disease resistance in birds. The flexibility of mRNA and vector-based vaccine platforms offers a scientific foundation for updating poultry vaccines much more quickly as new strains emerge. Automation and digital quality-control frameworks help scale the production of veterinary biologics with the consistency that large flocks require. Meanwhile, AI and modelling tools, originally built for clinical development and manufacturing decision, are being repurposed to predict outbreak patterns or identify early signs of risk across supply chains.
Bridging the Lab and the Farm
Translating these technologies into agricultural settings requires thoughtful adaptation. Tools developed in laboratory environments must be redesigned to work reliably in barns, hatcheries, and processing plants. Vaccine platforms need to balance scientific sophistication with practical realities such as cost, volume, and ease of administration. Digital systems must manage data from multiple environments; environmental sensors, lab results, movement logs, and farm records, to form a coherent early-warning picture. In each case, the principles that have guided progress in cell and gene therapy become stepping stones toward a new, more integrated approach to animal health.
Building Resilience in Food Systems
The ultimate purpose of this cross-sector translation is straightforward: to create agricultural systems that can anticipate, absorb, and recover from infectious threats with far greater precision and speed. Faster detection of high-risk viral strains gives farmers more time to take action. Updated vaccines that can be adjusted rapidly help protect flocks as the virus evolves. Stronger genetic and management insights contribute to longer-term resilience. Predictive digital platforms support smarter, earlier interventions. Together, these capabilities build a more stable food supply chain, one less prone to sudden shocks that disrupt livelihoods and traditions.
Introducing the Farm-to-Lab Biosecurity Flow Map
To make this emerging landscape intuitive and accessible, Lonrú has developed an interactive Farm-to-Lab Biosecurity Flow Map. It visually traces how enabling technologies originating in the cell and gene therapy ecosystem move through a translational pipeline and ultimately contribute to improved resilience on farms. The map provides a clear, structured way to explore how scientific innovation shapes everything from surveillance to vaccine development to outbreak prediction.
It also reflects Lonrú’s broader mission: to illuminate complex intersections between biotechnology, industry, and society, and to help enabling-technology companies understand where genuine opportunities exist beyond their original markets. As avian influenza continues to challenge agricultural systems in Ireland, the United States, and beyond, this perspective becomes increasingly valuable.
Explore the Interactive Flow Map Below
Click on each element of the map to see how specific technologies, translational steps, and on-farm outcomes connect to one another. The visual is designed to help you understand how innovations from the cell and gene therapy space can contribute to a more resilient approach to agricultural biosecurity.
Are You Post-Approval Ready? What the FDA’s New Guidance Means for CGT Enabling Technology Providers
Earlier this month, the FDA released its draft guidance on post-approval methods for collecting safety and efficacy data for cell and gene therapies. For many in the field, this felt like an incremental regulatory update. But for those who have followed our recent Lonrú Lens analyses, the shift fits a pattern we have been tracing for months. Our Evidence and Access Navigator mapped how post-approval data expectations in Europe and the United States are tightening. Our CRISPR Clinical Trials Dashboard highlighted the growing need for long term durability data across emerging gene editing programs. Our insights on the evolving UK ATTC and point-of-care manufacturing ecosystem showed how identity and batch data must persist across years of follow-up. And our Investment Signal Heatmap made clear that capital is flowing toward platforms that can prove durability and long term safety, not just early clinical success. The new FDA draft guidance brings all of these threads together. It elevates the importance of long term evidence and makes clear that enabling technology providers will be central to generating, structuring, and sustaining that evidence over the full lifecycle of a CGT product.
Most early reactions to the guidance focused on what it means for sponsors, but a more important shift is happening underneath the surface. Enabling technologies are becoming central to how long term evidence is shaped and maintained. At Lonrú, we see this guidance as the moment when enabling technology providers begin to play a more strategic role in defining the durability, safety, and comparability narrative that accompanies a therapy well beyond initial approval.
FDA’s message is clear: For CGTs, post-approval evidence is part of the product itself. Sponsors must be able to demonstrate ongoing safety, durability, manufacturing comparability, and patient level follow-up for as long as the therapy is on the market. For many products, this extends out 5, 10, even 15 years. No sponsor can maintain this level of data integrity alone. They depend on the platforms, orchestration systems, QC and potency tools, analytics technologies, registry-compatible datasets, and patient outcome solutions built by enabling technology providers.
This shift changes how companies in this space can differentiate. Enabling technologies have traditionally been seen as the infrastructure that keeps cell and gene therapy programmes moving. They manage the workflow, the scheduling, the identity, the metadata, the QC, the batch analytics, or the data capture. They remain essential in those roles. But the FDA guidance adds a new dimension. A platform is no longer judged only on the efficiency it brings to development or manufacturing. It is also judged on how well it contributes to the evidence the sponsor must ultimately defend.
Many enabling technologies already sit close to these evidence streams without framing themselves that way. Orchestration platforms hold identity events that can support long term data linkage. QC and CMC technologies maintain structured batch histories that help correlate changes in process with changes in clinical outcomes. Analytics engines convert raw clinical and real world data into harmonised datasets that align with regulatory expectations. Patient outcome tools become natural anchors for long term follow-up. Even companies whose tools sit entirely on the manufacturing or data layer still contribute critical information that supports evidence continuity.
The FDA has not asked enabling technology providers to reinvent themselves. It has simply clarified the importance of the capabilities they already provide. What is missing is a structured way for these companies to understand how their technology aligns with this new evidence environment and where further development or integration would create commercial advantage.
To help with this, we created the VantagePoint™ Post-Approval Evidence Readiness Scorecard. It is designed specifically for enabling technology companies and reflects the evidence domains highlighted in the new guidance. Instead of assuming all companies operate in the same space, the Scorecard begins by asking users to identify the archetype that best describes their technology. A manufacturing or CMC platform will naturally be strongest in different areas than a patient data system or an analytics engine. The Scorecard accounts for that by adjusting how each domain is weighted and how the overall readiness score is calculated.
The output is clear and practical. Users receive a weighted score that reflects what they can realistically influence, an assessment that highlights their strongest areas, and targeted recommendations for where strengthening or partnering could create the most strategic uplift. The aim is not to present a standard that all companies must match. Instead, it offers a realistic and forward-looking view of how each company already contributes to the evidence landscape and where new value can be created as expectations grow.
From Lonrú’s perspective, this is the moment for enabling technology providers to step into a more strategic role in the CGT ecosystem. Companies that understand how they support post-approval evidence will be the ones sponsors rely on as therapies progress from early trials into long term real world use. Their technologies will not only support operations, but will help secure the long term success of the therapies themselves.
You can explore the VantagePoint™ Post-Approval Evidence Readiness Scorecard below. It only takes a few minutes and provides a clear perspective on how your technology aligns with the evidence needs that will define CGT development in the years ahead. If you would like to discuss your results or explore how your platform can strengthen its position in this evolving environment, Lonrú would be glad to help.
How Public-Private Partnerships Are Shaping Global Cell & Gene Therapy
In advanced therapies, progress rarely happens in isolation. Cell and gene therapy (CGT) depends on infrastructure, collaboration, and regulatory certainty - all of which are too complex, costly, and strategically vital for any single actor to build alone. That is where public-private partnerships (PPPs) step in: alignment engines that share risk, accelerate translation, and anchor long-term manufacturing and data capacity. When structured and executed well, PPPs become more than funding mechanisms - they define the shape of the industry that follows. They create the training pipelines, supply networks, and translational ecosystems that turn one-off breakthroughs into reproducible therapies.
Why PPPs Matter in CGT
Every new therapy requires more than an idea: it needs clean rooms, viral vectors, automation systems, analytics, registries, and people trained to operate them. PPPs are how that invisible infrastructure gets built. Governments de-risk capital investment. Academia and hospitals supply translational expertise. Industry brings scalability, digitalization, and commercial discipline. When these forces align, they generate a multiplier effect - regional capability, investor confidence, and faster access for patients.
Introducing the VantagePoint™ CGT Public-Private Partnership Tracker
To map this alignment, Lonrú has compiled and analysed 16 major PPPs announced between 2019 and 2025, covering North America, Europe, Asia-Pacific, the Middle East, and Latin America.
These include high-profile initiatives such as:
AMP Bespoke Gene Therapy Consortium - USA (Rare Disease Program)
Tailored Genes - Canada FedDev (Viral Vector Expansion)
ARC Hub - Ireland ERDF (All-Island Therapeutics Initiative)
OmniaBio - Canada ISED (Commercial CGT Facility)
Berlin Center for Gene and Cell Therapies - Germany (Integrated R&D Hub)
GEMMABio & Fiocruz - Brazil (Gene Therapy Manufacturing Partnership)
Together, they represent more than USD 1 billion in disclosed investment dedicated to building CGT infrastructure, automation capability, and workforce readiness.
What the Tracker Shows
Global momentum
Since 2023, PPP activity has accelerated sharply. Governments from Ireland to Saudi Arabia have launched multi-year programs to localize CGT capacity, shifting emphasis from research to manufacturing resilience.
Manufacturing dominance
Nearly every partnership includes a manufacturing or supply-chain component - from vector production (Canada, Germany) to AI-driven process automation (OmniaBio) and GMP workforce programs (UK BEIS / CGT Catapult). For enabling-technology vendors, this signals sustained demand for automation, analytics, and digital QC solutions.
Regional diversification
Europe and North America still lead, but Asia-Pacific and the Middle East are expanding fast.
Singapore’s A*STAR-SCG Cell Therapy collaboration and Saudi Arabia’s KFSHRC facility underline how new manufacturing corridors are forming outside the traditional biotech capitals.
Vendor-signal patterns
Across all records, Manufacturing / Supply Chain appear in more than 80 percent of cases; Analytics in 60 percent; and Workforce Development in 20 percent. The pattern is clear: global capacity building now extends beyond facilities to skills and data - a direct opportunity zone for Lonrú’s enabling-technology clients.
Inside the Tool
The new VantagePoint™ CGT Public-Private Partnership Tracker brings this dataset to life through an interactive interface built on Google Cloud Run and powered by Lonrú’s analytical framework.
Key features
Global map and timeline - Plot PPPs by geography, investment value, and modality; explore temporal trends from 2019 to 2025.
Filter controls - Slice the data by continent, region, modality, vendor signal, or investment range.
Sortable table view - View, search, and export partnership details (partners, objectives, investment, and verified source links).
Dynamic charts - Visualize investment flows by region and vendor-signal category with interactive tooltips.
AI Insight Generator - Powered by Gemini through Google AI Studio, this feature analyzes whichever view the user is exploring (filtered or global) and generates 2-3 concise, evidence-based insights.
With no filters applied, it produces a global overview.
When filters are active, it delivers context-specific intelligence (for example, “APAC manufacturing momentum” or “RWE infrastructure gaps in Europe”).
Each insight is classified under a VantagePoint™ Lens - INSIGHTS, CONNECT, ACCELERATE, VALIDATION, SCALE, or ILLUMINATE - mirroring Lonrú’s consulting framework.
These features transform the tracker from a static register into a dynamic intelligence environment - a living map of where collaboration and capital are building the next phase of CGT.
Explore the Data
The full dataset and interactive tool are embedded below.
Use the filters to focus on your region or vendor category, then review the AI-generated insight cards to see what the data implies for your business.
VantagePoint™ CGT Public-Private Partnership Tracker
A VantagePoint™ INSIGHTS Product
The CGT Public-Private Partnership Tracker sits within Lonrú’s VantagePoint™ INSIGHTS suite - tools that convert regulatory and market complexity into actionable commercial foresight.
By illuminating the partnerships building tomorrow’s therapy infrastructure, it helps enabling-technology companies see where capacity, collaboration, and opportunity will converge next.
From Europe’s Evidence Framework to the U.S. Commercial Opportunity in CGT
Last week in our Lonrú Lens we explored how Europe is redefining post-approval evidence and market access for cell and gene therapies through our interactive continent-wide tool. That article guides enabling-technology providers through the complex national HTA, registry and long-term follow-up requirements across the UK, Germany, France and Italy.
This week, we turn our attention to the United States. While Europe emphasises harmonisation and alignment, the U.S. remains the global bellwether for where commercial and technical demands land first. With almost two-dozen CGT approvals under the belt, the VantagePoint™ Cell & Gene Therapy Evidence & Access Navigator now maps how each U.S. therapy brings a tail of obligations - obligations that translate directly into opportunities for enabling-technology providers.
Introducing the U.S. View: The Evidence & Access Navigator for Cell & Gene Therapy
Our U.S. edition of the Navigator captures every U.S. approval from the first CAR-T therapy in 2017 through the latest 2025 entry. Each record links modality, indication, manufacturing constraints and post-market commitments to a set of “vendor signals” - concrete indicators of where analytics, manufacturing, logistics, diagnostics and patient-support vendors will be needed. By doing so, enabling-technology companies can anticipate what will be built next and plan with purpose.
Why This Matters for Enabling-Technology Providers
Every approval isn’t just a win for a therapeutic sponsor - it’s a signal for the ecosystem. Future-proof tools don’t just respond to science; they align with the administrative, regulatory and delivery burdens that follow.
If your company provides QC or analytics platforms, the key question isn’t “What therapy got approved?” but “What is the comparability or potency-data demand that results from this approval?” If you specialise in RWE or registry services, you’ll want to know which products carry 15-year safety or durability commitments. If you build logistics or cryostorage systems, you’ll ask “Which approvals require autologous chain management or temperature-sensitive vector delivery?”
The Navigator makes those questions answerable. Instead of chasing vague trends, you can shape your product roadmap around real obligations - giving you line of sight into where sponsors will spend next, and where your enabling technology fits.
What the Data Shows
The U.S. dataset illustrates that the enabling-technology market is entering a phase of sustained demand, not just one-off launches. Across the 19 therapies captured:
Manufacturing complexity appears in every one of them—confirming that robust process, vector/cell supply, and comparability infrastructure remain foundational.
RWE and long-term follow-up commitments appear in fourteen therapies, signalling that real-world platforms and outcomes tracking are now table stakes.
Analytics obligations similarly stretch across fourteen therapies—highlighting that data-interpretation and assay-readout engines remain a key service layer.
Supply-chain/logistics burdens surface in twelve therapies—particularly for high-dose AAV, autologous harvest, or cold-chain sensitive administration.
Patient-support and diagnostics are less frequent, but highly strategic, especially for new modalities such as gene editing, ocular delivery, or topical therapy.
These patterns tell a clear story: the market is shifting from therapy-by-therapy “first-in” launches toward infrastructure-enabled scale. The tools that deliver continuity, data clarity and execution speed will define the next era of CGT.
How to Use the Navigator
The Navigator is built with enabling-technology providers in mind. You’ll begin by filtering by Modality, Year, or Vendor-Signal to zoom into your space of interest - whether that’s AAV manufacturing, RWE platforms or next-gen diagnostics. Then, by expanding a therapy row, you’ll see its commitments and vendor-signal cue-words - giving you clarity on who needs what next.
Users can export the data and embed or annotate it in your internal strategy processes without worrying about shifting inputs. If you’d like a focused slice, or expand to include VantagePoint™ - say, all 2024 AAV approvals with supply-chain complexity - we can tailor that for you.
Explore the Data
VantagePoint™ Cell & Gene Therapy Evidence & Access Navigator
A VantagePoint™ INSIGHTS Product
The VantagePoint™ Cell & Gene Therapy Evidence & Access Navigator sits firmly within Lonrú’s Insights suite - where we convert regulatory and market intelligence into actionable commercial foresight. For enabling-technology companies, it offers a vantage point into where capability gaps turn into growth opportunities.
Mapping the Approval Landscape of Europe’s Cell & Gene Therapies
Europe is leading the charge in defining how advanced therapies are approved, reimbursed, and monitored after they reach patients. But until now, these post-approval rules have been scattered across dozens of national HTA decisions and regulatory annexes. Our new Cell & Gene Therapy Evidence & Access Navigator brings it all into one view - showing how each therapy, across the UK, France, Germany, and Italy, is linked to the evidence that sustains its market access.
Seeing the full picture
The Navigator, shown below, allows users to explore 70 therapies authorised or reimbursed in Europe. Each card links to its underlying regulatory and HTA documentation, tracking how obligations evolve across countries. The goal is simple: to make it easier to see where Europe’s post-approval frameworks are converging and where national variations are emerging.
1. Evidence is now a long-term contract
Almost every authorised CGT in Europe carries a 15-year long-term follow-up requirement. Our heatmap makes this clear: across all four major markets, over 90 % of products are monitored through formal LTFU programmes. For AAV-based therapies like Roctavian and Zolgensma, this means routine immunogenicity tracking and durability assessments. For autologous CAR-T and CRISPR therapies, it extends to comparability data whenever manufacturing changes occur.
2. Registries are becoming Europe’s data backbone
The Navigator’s country lens shows that:
Italy leads with fully operational AIFA registries - Breyanzi, Abecma, Carvykti, Yescarta, and Kymriah all run under registry-linked reimbursement.
Germany embeds evidence collection into AMNOG AbD registries, requiring structured post-market data for therapies like Zolgensma and Roctavian.
The UK uses Managed Access Agreements through NICE - gathering outcomes data within defined cohorts.
France remains the most traditional, granting reimbursement after HAS opinions but increasingly layering real-world data commitments on top.
Together, these systems are transforming registries from administrative tools into evidence currencies - the means by which therapies maintain reimbursement over time.
3. Patterns across modalities
Our Insights Dashboard aggregates the data across all therapies:
21 % of products have mandatory registries today - a figure expected to rise as newer approvals enter AIFA and G-BA systems.
91 % have ≥ 15-year follow-up obligations.
84 % carry explicit comparability data requirements.
Across modalities:
In vivo AAV products dominate immunogenicity monitoring.
Ex vivo autologous therapies (CAR-T, CRISPR, lentiviral) anchor the registry-linked access models.
Allogeneic therapies such as Ebvallo show that even donor-cell platforms now fall under long-term oversight.
4. What this means for enabling-tech providers
For the tools and technology companies Lonrú supports, Europe’s CGT evolution creates new commercial signals:
Evidence readiness is market readiness.
Launch success now depends on how well tools integrate with national registries and data standards.
Comparability analytics are a growth driver.
As regulators demand proof of consistency between manufacturing runs, there’s an expanding market for AI-driven release and process-monitoring platforms.
Data interoperability is the next competitive edge.
Enabling-tech firms that can bridge clinical, manufacturing, and payer data will lead Europe’s next phase of CGT industrialisation.
5. A VantagePoint™ for European alignment
By consolidating data from the EMA, NICE, HAS, G-BA, and AIFA, the Navigator provides a single vantage point to watch Europe’s evidence frameworks evolve in real time. Patterns that once required hours of cross-referencing are now visible in seconds.
Explore the Navigator Below
Visitors can filter by therapy, country, or modality; switch to the Evidence Obligations Heatmap to compare follow-up and registry intensity; or dive into the Insights Dashboard to see how obligations cluster across the continent.
About the Navigator
The CGT Evidence & Access Navigator is powered by Lonrú Consulting’s verified dataset (v12_master), built entirely from public sources:
EMA European Public Assessment Reports
NICE, HAS, G-BA, and AIFA decisions and registries
It’s part of our ongoing work to illuminate how post-approval evidence systems shape the future of advanced therapies in Europe.
How Advanced Therapy Treatment Centre Networks Enable National Delivery of Cell and Gene Therapies
As cell and gene therapies move from scientific promise to clinical reality, the challenge for every health system is shifting from innovation to implementation.
How do you make these complex, patient-specific treatments accessible at scale - not in isolated pilot sites, but across an entire national network?
The UK’s Advanced Therapy Treatment Centre (ATTC) model offers one of the most instructive answers to that question.
By coupling regional infrastructure with coordinated national governance, the ATTCs have created a practical framework for how countries can scale up and out advanced therapies - transforming clinical potential into real-world delivery.
Now entering its seventh year, the network provides a valuable reference point for any country seeking to build a sustainable ecosystem for advanced therapy manufacturing, logistics, and care delivery.
Lonrú Consulting’s ATTCs at Scale project analyses how the network is performing today - and what it reveals about the future of distributed innovation in cell and gene therapy.
Using Lonrú’s curated dataset and the interactive ATTCs at Scale data tool (embedded below), we’ve mapped how more than 120 UK advanced therapy trials connect to the four regional hubs: iMATCH (Manchester), Midlands & Wales ATTC, Northern Alliance ATTC, and London ATTC.
From Dataset to Dashboard
Our analysis draws from curated data sourced from ClinicalTrials.gov, covering active and recently completed UK trials through 2024.
Each trial in the dataset has been mapped to one or more ATTC nodes using its hospital sites and institutional affiliations.
Where a study spans multiple ATTC regions, participant numbers have been divided evenly across nodes for visualisation purposes. These allocations have not been verified directly with ATTC teams and should be treated as indicative rather than audited figures.
The tool transforms this dataset into four interactive views:
1️⃣ Interactive Map
Explore the UK’s ATTC network geographically.
Each regional hub appears as a node on the map - click to reveal:
Total trials hosted at that node
Estimated participant counts
Leading modalities
Top sponsors and collaborators
Example: iMATCH (Manchester) currently shows 27 mapped trials and approximately 991 participants, with activity spanning T-cell therapies, lentiviral gene transfer, and early AAV programmes.
2️⃣ Trial Composition
This view compares trial counts by modality type across ATTC nodes.
Bars represent therapy categories such as cell therapy, ex vivo gene therapy, in vivo gene therapy, and gene editing.
Users can see at a glance that:
London ATTC dominates overall trial volume (~80 active studies), reflecting the density of sites across Guy’s, GOSH, and UCLH.
Midlands & Wales and Northern Alliance host smaller but growing portfolios, concentrated around translational manufacturing and readiness pilots.
iMATCH retains a strong cell therapy bias, with nearly half its mapped trials classified as T-cell based.
3️⃣ Node Scorecards
Each ATTC has its own performance card summarising:
Total mapped trials
Estimated participant count
Leading therapy modalities
Key sponsors active at that node
For instance, London ATTC shows 81 trials and roughly 4,476 participants, led by AAV gene therapy, T-cell therapies, and oncolytic vectors.
This structure makes it easy to benchmark regional specialisation and sponsor clustering.
4️⃣ Modality Distribution Heatmap
The heatmap aggregates proportional modality share across all four hubs.
Each cell represents a node-modality intersection, shaded by its relative weight.
Patterns stand out immediately:
London balances gene and cell therapies.
Midlands & Wales concentrates on viral vector programmes.
iMATCH is heavily weighted toward T-cell work.
Northern Alliance displays a more even, early-phase spread.
Methodology Summary
Source: ClinicalTrials.gov (public registry, data cut-off January 2025).
Selection: UK-based interventional CGT/ATMP studies across Phase I–III.
Classification:
Trials assigned to ATTCs by hospital/institution field.
Modalities tagged using intervention text (e.g., “CAR-T”, “AAV”, “lentiviral”, “CRISPR”).
Estimates: Where a trial spans multiple ATTCs, participant numbers were divided evenly across nodes.
Verification: Data have not been validated directly with ATTC centres or trial sponsors.
This approach prioritises consistency and transparency over precision - giving an accurate macro-view of the UK network while acknowledging site-level variation.
Disclaimer
These data are sourced from ClinicalTrials.gov and represent a public-domain snapshot of registered studies as of 2025.
Figures presented here - including trial counts, patient estimates, and node allocations - are derived from registry information and have not been verified with ATTC teams or study sponsors.
Participant distributions across multiple sites are evenly apportioned for modelling purposes only.
Explore the Tool
The interactive dashboard below - ATTCs at Scale - brings this analysis to life. Dive into the map to explore individual centres, switch to composition charts to compare modalities, or use the heatmap to understand proportional strengths across the network. Each view represents a snapshot of how the UK’s advanced therapy ecosystem is scaling - quietly, collaboratively, and region by region.
Closing Insight
The ATTC network has matured from initiative to infrastructure, quietly underpinning how advanced therapies reach patients across the UK. By linking clinical readiness with manufacturing and governance, the network has become the functional interface between research ambition and patient delivery. As global attention moves from discovery to delivery, this network provides a model of what coordinated, data-driven translation can look like - and why infrastructure, not just innovation, defines impact.
Europe’s Next Translation: Mapping the Networks Behind Cell and Gene Therapy
As the industry gathers in Seville for ESGCT 2025, the conversation is turning toward scale, connectivity, and what comes next for Europe’s cell and gene therapy field (CGT). At Lonrú Consulting, we wanted to look across the ecosystem to understand where strength already exists, where new capability is forming, and where opportunity lies.
To do that, we built the Translational Network Atlas.
The Atlas is a data-driven visualisation of Europe’s CGT landscape. It brings together comparable metrics on translational infrastructure, manufacturing capability, scientific output, and cross-border collaboration for sixteen European countries. Each country is represented by a Composite CGT Readiness Score, built from Lonrú’s underlying Collaboration and Infrastructure Dataset.
The goal is simple: to see the European CGT ecosystem as it really functions, not as disparate national efforts but as a connected system of translational engines.
About the Methodology
Each country’s Composite Score represents the weighted average of five underlying factors that describe its cell and gene therapy (CGT) ecosystem:
European Network Integration – how connected the country is to major European translational frameworks and consortia
Translational Platform Maturity – how well-developed its clinical and regulatory infrastructure is
ATMP Manufacturing and Execution Capacity – the scale and readiness of its GMP and technology transfer capabilities
Cross-Border Collaboration Participation – how active it is in regional or multi-country initiatives
CGT Publication Intensity – how much peer-reviewed research output the country contributes to the field
Each metric is scored from 0 to 5 and weighted by importance. Under the Balanced preset:
Integration: 25%
Maturity: 25%
Manufacturing: 20%
Collaboration: 15%
Publications: 15%
The Calculation
For a country c, with metric values I, M, F, C, and P (each between 0 and 5) and weights wI, wM, wF, wC, and wP (expressed as percentages), the Composite Score is calculated as:
This is a weighted average of the five metrics on a 0–5 scale.
Example (illustrative only)
If a country has Integration = 5, Maturity = 4, Manufacturing = 5, Collaboration = 2.5, Publications = 3.5, then under the Balanced weighting:
= 4.15
Note: These values are for demonstration only and do not represent live scores.
Why It Matters
The weighting tells the index what to prioritise. Policy-focused users might emphasise Integration and Maturity; industry users might give more weight to Manufacturing; academic users might focus on Collaboration and Publications. The same formula applies no matter the weighting - it simply reflects a different perspective on the same ecosystem data.
What the Atlas Reveals
The early findings show a continent defined by complementarity rather than competition.
The UK, France, and Germany form Europe’s industrial and translational core, linking advanced manufacturing with regulatory sophistication.
The Netherlands, Spain, and Italy bridge academia and execution, combining strong research output with GMP expansion.
The Nordic cluster stands out for its network-driven collaboration model, while Ireland, Portugal, and Austria are investing strategically to accelerate translational maturity.
Across all regions, integration is deepening. Europe’s collective CGT capacity is becoming more networked, more balanced, and more visible.
Explore the Atlas
The Translational Network Atlas is available to explore below where users can move across the global map, compare national CGT readiness profiles, and see how integration, maturity, and knowledge interact to shape the future of advanced therapy translation. This tool is not a ranking but is instead a window into how Europe’s CGT engine operates and where the next translational opportunities may appear.
For custom research and data visualisation tools contact Lonrú Consulting.
2025 Investment Signal Heatmap: A Snapshot of Cell and Gene Therapy
The cell and gene therapy field continues to evolve. Alongside breakthrough science, 2025 has brought both new capital inflows and painful workforce reductions. To make sense of the mixed signals, Lonrú Consulting has built an Investment Signal Heatmap.
This interactive tool captures a fixed snapshot of publicly reported financings, acquisitions, layoffs and shutdowns across 2025. Each cell in the matrix shows a net signal score, balancing investment against contraction, and lets you click through to see the events behind the number.
What the matrix shows
The matrix consolidates the data into four modality families, to capture the essential scientific groupings that investors, developers and enabling companies all track:
Cell therapies (covering CAR-T, TCR and NK approaches)
Gene therapies - viral (AAV, lentiviral and related vectors)
Gene editing (CRISPR, base, prime and epigenetic editing)
RNA and non-viral (mRNA/LNP and emerging non-viral delivery systems)
Against this we set three categories that reflect the types of technologies and infrastructure that support every program:
Delivery and engineering (electroporation systems, LNPs, viral vector engineering, upstream instrumentation)
Analytics and assays (QC, potency, off-target measurement, research tools)
Manufacturing and regulatory (CDMOs, software, clinical and regulatory operations)
This structure keeps the matrix compact and offers clarity. It also reflects how capital actually flows: money moves into therapeutic modalities on one axis and into the enabling tool and service layer on the other.
We then defined strict criteria for what counts as a signal. Only investment and workforce events were included; financings, acquisitions, asset sales, partnerships with disclosed financials, and layoffs, divestments or shutdowns. We deliberately excluded softer announcements like product launches or new assays unless they were tied to funding or jobs. That way every cell is lit by hard evidence of capital flowing in or out.
Scores are calculated on a log scale so that magnitude matters. A billion-dollar acquisition should outweigh a small seed round, and a thousand-person layoff should weigh more heavily than a dozen redundancies. Stage weights give more influence to later-stage financings, and severity weights reflect the difference between minor and major reductions. The outcome is a single net score per cell that balances positive and negative events, providing a quick visual read on where the field is expanding and where it is under pressure.Main trends
Gene therapies - viral are the bright spot. The field shows the strongest positive signal in 2025, driven by large raises at Kriya and Atsena and major CDMO financings from WuXi AppTec and Oxford Biomedica. These outweighed Bluebird’s cash crunch and layoffs at Catalent.
Gene editing is mixed. Big pharma’s $1.3 billion acquisition of Verve and a $175 million raise by Tune Therapeutics were significant positives. But shutdowns at Spotlight, layoffs at Prime Medicine, and retrenchments in enabling companies like MaxCyte and Eikon left the overall balance just above neutral.
Cell therapies are under pressure. Arsenal, Allogene and Nkarta all made substantial staff cuts. Adaptimmune’s $55 million asset sale offered a counterweight but did not offset the contraction.
RNA and non-viral is flat to negative. Vertex’s $65 million partnership with Orna added a positive note, but Moderna’s 10 percent workforce cut, and layoffs at suppliers TriLink and Aldevron, weigh the score into negative territory.
Why this matters
For investors, partners and operators, the Heatmap makes it clear where momentum is building and where caution is needed. It also surfaces enabling technologies that may not make headlines but are critical to the health of the ecosystem.
Explore the Heatmap
The Investment Signal Heatmap is available to explore below. Click into any cell to see the events and analysis behind each score.
This is a snapshot, not a forecast. But it provides a clear window into how capital is shaping the cell and gene therapy field right now, and where the opportunities and risks are emerging.
RNA x Cell and Gene Therapy: exploring the Intersections
RNA has stepped from the shadows of vaccine development into the spotlight of next-generation cell and gene therapies. What was once viewed primarily as a rapid-response vaccine modality is now being applied to some of the most complex bottlenecks in cell and gene therapy (CGT) - delivery, safety, scalability, and flexibility.
At Lonrú, we wanted to look systematically at where RNA is already making an impact in CGT, and where the emerging evidence points to new opportunities. The result is an interactive Explorer that maps how RNA technologies intersect with CGT across payload types, delivery systems, and the companies pioneering these approaches.
Why RNA matters in CGT
The unique attributes of RNA make it an enabling technology in multiple ways:
Transient expression: Unlike DNA-based vectors that may persist long term, RNA expression is short-lived. This offers an alternative approach where temporary protein production or cell engineering is desirable, and it can allow for repeat dosing strategies.
Engineering flexibility: Ex vivo, mRNA can reprogram immune cells such as CAR-T or NK cells without the need for viral vectors, streamlining manufacturing.
Editing delivery: Co-delivery of editor mRNA with guide RNAs has become the backbone of in vivo CRISPR approaches, demonstrated clinically in Intellia’s NTLA-2001 program.
Expanding modalities: Self-amplifying RNA (saRNA) and circular RNA (circRNA) extend durability and stability, broadening therapeutic possibilities.
What the data shows
The Explorer collates citable evidence across peer-reviewed publications, clinical trial records, and company disclosures. Key takeaways from the Delivery × Payload Matrix include:
Electroporation + mRNA remains a clinical workhorse, with multiple CAR-T programs trialled using transient mRNA engineering.
LNPs + mRNA+gRNA dominate in vivo editing strategies, from NTLA-2001’s ATTR program (NEJM 2021) to recent prime editing demonstrations.
Custom LNPs designed for immune cells are emerging, pushing RNA delivery beyond the liver into T cells and other compartments.
Polymeric nanoparticles offer early preclinical signals as an alternative to LNPs, but remain less developed.
saRNA and circRNA are present largely at the platform and early translational level, with limited but growing relevance to CGT.
Explore the data
Within the Explorer, you can:
Cross-filter by payloads, delivery systems, or sponsor type.
Drill down into the Delivery × Payload Matrix, with linked examples that connect directly to publications, trial records, or company science pages.
Spotlight BioNTech and Moderna, whose RNA platforms are shaping new intersections with CGT.
Download the Lonrú curated Sources Table for further reading and reference.
Trace partnerships through the network view, with active, paused, and terminated collaborations all clearly mapped.
Why we built it
Our mission at Lonrú Consulting is to bring clarity to enabling technologies in CGT. By collating disparate data points into a single, interactive view, we aim to illuminate how RNA is already shaping the field - and where opportunities for new enabling partnerships lie.
Scroll down to explore the intersection between RNA and Cell and Gene Therapy.
Ireland’s Cell and Gene Therapy Map: Illuminating the Island’s Growing Ecosystem
Introduction
Last week’s launch of the Irish Society for Gene and Cell Therapy (ISGCT) website marked a milestone in building visibility for advanced therapies across the island of Ireland. It comes at a time when Ireland’s ecosystem - spanning research, clinical delivery, and manufacturing - is accelerating rapidly.
At Lonrú Consulting, we see this as the right moment to shine a light on the opportunities Ireland presents for enabling technology providers. To make this landscape more tangible, we’ve built a new resource: Ireland’s Cell and Gene Therapy Map - an interactive tool that brings the island’s ecosystem into focus.
Ireland’s Cell and Gene Therapy Map
Ireland’s Cell and Gene Therapy Map is a first-of-its-kind interactive ecosystem map showcasing:
Research centers advancing discovery and translational science.
Clinical sites delivering stem cell transplants and CAR-T therapies.
Manufacturing facilities producing GMP-grade advanced therapy products.
Key players and consortia driving collaboration and innovation.
Explore the interactive map below.
The tool enables users to click through academic hubs, GMP facilities, consortia, and companies, discovering why each node matters for tool providers. Filters make it easy to focus on capabilities such as GMP, analytics, non-viral delivery, or clinical trial readiness.
Why It Matters for Enabling Technology Providers
The map demonstrates that Ireland offers more than clinical delivery or traditional biopharma manufacturing. From Takeda’s stem cell manufacturing facility in Dublin, to CCMI’s GMP-licensed ATMP site in Galway, to St. James’s CAR-T program, and HiTech Health’s new CDMO capacity, Ireland is becoming a proving ground for enabling technologies.
For providers of analytics, automation, delivery platforms, and quality systems, Ireland is a place to:
Pilot tools with translational centers and GMP labs.
Validate technologies in live manufacturing and clinical environments.
Partner with consortia like ISGCT and Can-Vas to link into funding and clinical trials.
Lonrú’s Perspective
With Ireland’s Cell and Gene Therapy Map, we’ve built a resource that not only catalogs facilities, but highlights why each node matters for tool and platform providers.
This aligns with our ethos: Navigating complexity, illuminating innovation. By combining insights, interactivity, and data-driven clarity, we aim to help enabling technology providers see Ireland’s position in the global CGT landscape.
Looking Ahead
With the relaunch of ISGCT and the launch of our interactive map tool providers can see that Ireland is ready to step forward as a connected hub for cell and gene therapy.
For enabling technology providers, the opportunity is clear: engage early, validate locally, and scale globally.
CRISPR Clinical Trials: Global Shifts and New Directions
At Lonrú Consulting, we believe clarity comes from illumination. That’s why we’re excited to introduce our newest interactive tool: the VantagePoint™ CRISPR Clinical Trials Dashboard. Built by Lonrú Studios™, our custom analytics and visualization arm, the dashboard transforms raw data into insight - helping our clients see where the field is heading, and why it matters for enabling technologies.
What the data shows
By exploring the latest dataset of CRISPR-focused clinical trials, we can see both where research is accelerating globally and the new directions shaping the next wave of gene editing:
Therapeutic momentum is shifting
The majority of ongoing CRISPR trials are concentrated in oncology and hematology, but the pipeline is diversifying. We’re now seeing increasing activity in metabolic indications, as well as ophthalmology and rare genetic disorders.Ex vivo editing remains dominant, but boundaries are moving
Most active programs continue to leverage ex vivo approaches (particularly in T-cell and hematopoietic stem cell therapies). However, a growing number of early-phase trials are testing direct delivery platforms - signalling opportunity for providers of delivery vectors, lipid nanoparticles, and targeting systems.Global expansion is accelerating
Many assume the US is at the heart of CRISPR clinical development, but the data tells a different story. China now leads in sheer volume of CRISPR trials, outpacing the US and Europe. It’s no coincidence that some of the biggest bio-pharma partnerships announced this year have been sourced in China. For enabling technology providers, this underlines the strategic importance of engaging with Chinese innovators and preparing for globally distributed development pathways.Emerging trends in editing technologies
The field is no longer just underpinned by CRISPR-Cas9. We see exploratory trials beginning to reference base editing and prime editing, pointing to a diversification of platforms that will demand new supporting tools, analytics, and quality frameworks.
What’s new in the past month?
The trials updated in the past month offer a good snapshot of these new directions; from late-phase genetic programs to unconventional nutrition-linked studies:
Vertex Pharmaceuticals has registered a new Phase 3 program (NCT05951205) focused on a single-dose intervention in genetic disease. This underscores how established leaders are moving rapidly toward pivotal studies, reflecting both confidence in the science and a push for near-term approvals.
Haikou Affiliated Hospital of Central South University (China) has initiated an in vivo CRISPR trial targeting airway basal stem cells (NCT07018804). While still small in scale, it highlights China’s continued leadership in first-in-human in vivo approaches.
Quadram Institute Bioscience (UK) has launched a non-classical intervention trial (NCT07142759) exploring Vitamin D biofortified foods in a CRISPR trial context. While not a traditional therapeutic editing program per se, it signals a growing interest in how adjacent lifestyle and nutritional factors intersect with gene-editing research. This broadening scope is worth watching as it could open new categories of enabling technologies and cross-disciplinary collaborations.
Why this matters:
Together, these updates show that the CRISPR pipeline is diversifying in strategy. From pivotal late-phase trials to unconventional nutrition-linked studies, the field is probing both mainstream and novel directions. For enabling technology providers, this means opportunities will span beyond traditional vectors and QC tools, into areas where gene editing intersects with adjacent health domains.
Why this matters for enabling technology providers
These patterns carry direct implications for Lonrú’s clients:
Manufacturing & process innovators should anticipate increasing demand for scalable ex vivo workflows alongside flexible platforms capable of supporting in vivo expansion.
Analytics & QC tool developers have a growing opportunity to differentiate as editing modalities diversify.
Delivery technology providers are moving into sharper focus as sponsors test in vivo approaches.
In short: as CRISPR clinical trials broaden, the enabling ecosystem becomes the key to acceleration.
A tool to guide strategy
The new dashboard offers a clear vantage point. By surfacing shifts in indications, geographies, and editing approaches, it helps stakeholders identify where their solutions align with unmet needs and where strategic investment is headed.
At Lonrú Consulting, our mission is to empower the technologies behind CGT breakthroughs. Through VantagePoint™ Insights and custom dashboards from Lonrú Studios™, we illuminate complexity and help our clients act with confidence.
👉 Explore the dashboard for yourself below, or click here to view our full suite of interactive dashboards: VantagePoint™ Interactive Dashboards.
The Arc of Innovation in Cell & Gene Therapy: A Decade of Milestones and Signals Ahead
For much of the 2000s, cell and gene therapy was synonymous with viral delivery. AAV, lentiviral, and retroviral platforms defined the way the field engineered cells, delivered payloads, and approached manufacturing. These systems enabled the first wave of approvals and proved that genetic medicine could be real, but they also brought with them limitations—complex scale-up, safety concerns, and payload constraints.
The last decade has marked a clear shift. New modalities—non-viral, protein-only, mRNA-only, and in vivo approaches—have begun to reshape expectations for what therapies can look like and how they can be made. That is where our lens begins: in 2016, with the infusion of CRISPR-edited T cells. Not because it was the first milestone, but because it marked the start of a new era in which the dominance of viral vectors began to give way to a more diverse landscape.
Looking back, we can trace a clear progression:
2017 cemented viral validation with Luxturna and the first CAR-T approvals.
2020 redefined delivery as mRNA vaccines demonstrated at global scale that non-viral modalities could be safe, effective, and manufacturable.
2023 brought the first durable in vivo base editing data, showing that protein-only systems could drive meaningful clinical outcomes.
2024–2025 is set to be remembered for strategic bets: Interius entering the clinic with in vivo CAR-T, and AstraZeneca acquiring EsoBiotec to gain a foothold in reprogramming.
Each milestone tells its own story—but together they map a larger narrative: viral dominance giving way to a more diverse delivery landscape, and a steady march from ex vivo manipulation toward in vivo reprogramming.
Why this matters now
For enabling technology providers—the companies building the assays, platforms, and processes that make therapies possible—these shifts are not abstract. They determine where demand will concentrate, which capabilities will command premiums, and what regulators will expect as standards. The move toward protein-only, RNA-only, and in vivo modalities means that tools once designed for viral vectors are no longer sufficient.
Signals ahead
The next five years will test whether early promises translate into durable platforms.
By 2027, pivotal RNP-only programs in blood disorders could establish protein-only delivery as a credible alternative to viral editing.
By 2029, the first approvals of mRNA-only therapies in oncology and autoimmune disease, alongside in vivo CAR-T, would confirm that the delivery ecosystem has truly diversified.
Explore the narrative
We created The Arc of Innovation Timeline as a companion to this story - an interactive way to walk through the milestones, compare modalities, and see how today’s shifts set the stage for tomorrow’s breakthroughs. It reflects the philosophy behind our VantagePoint™ services: transforming complex data into clear, engaging insights that don’t just inform, but illuminate. For us, making data interactive is about helping enabling technology providers see the bigger picture and act with confidence.
Explore The Arc of Innovation Timeline below.
A Whistle-Stop Tour Around the Cell And Gene Therapy World
When you step back and take a truly global VantagePoint™, it’s remarkable how diversely and dynamically the cell and gene therapy (CGT) ecosystem is evolving. Different regions are leaning into their strengths: infrastructure, regulatory experimentation, workforce training, or cost disruption. For those building the enabling technologies behind CGT; the reagents, digital tools, automation platforms, and analytics, this creates a mosaic of opportunity.
At Lonrú Consulting, we wanted to give you a snapshot tour; east to west of the latest regional dynamics shaping CGT, and why they matter for tool providers. To make this more dynamic, we’ve built an interactive globe that lets you spin from Singapore to São Paulo, click into a region, and see the highlights in context. Scroll down to explore the journey and engage with the map.
Around the World: Regional Cell & Gene Therapy Highlights
How to explore
- Drag to rotate. Wheel or pinch to zoom.
- Click a pin to open details. Popups appear above pins.
- Use ⟵ ⟶ to step through regions. Auto View resets to AU/NZ.
Asia-Pacific
Australia
Australia is building sovereign CGT manufacturing strength. Facilities in Melbourne and Brisbane are focusing on viral vector supply and GMP cell manufacturing. Vendors in automated bioprocessing, digital quality control, and real-time analytics will find strong demand. Workforce training initiatives reinforce adoption of closed-system and scalable platforms.
New Zealand
New Zealand’s CGT presence is smaller but growing through clinical trial networks and government-backed biotech initiatives. Tool providers with portable manufacturing and data-light QC solutions can position early, especially as NZ leverages collaboration with Australian hubs.
Singapore
Singapore has established itself as a Southeast Asian CGT hub. Investments in ACTRIS, Biopolis, and REMEDIS are structured to help projects move smoothly from pilot scale to GMP. This creates appetite for MES systems, electronic batch records, and analytics tools that reduce tech transfer friction.
China
China is home to a rapidly expanding CGT pipeline across oncology, ocular, and metabolic programs. NMPA approvals are rising, which drives demand for genome editing platforms, delivery systems, and CMC characterization tools.
Japan
Japan’s conditional approval pathway accelerates regenerative therapies but requires real-world evidence. Vendors with decentralized manufacturing solutions, potency assays, and hospital-ready quality systems are well positioned.
South Korea
South Korea is scaling with policy support and new facilities. Growth projections exceed 15 percent annually, opening opportunities for automated expansion tools, comparability assays, and cold-chain logistics solutions.
India
India’s NexCAR19, the first indigenous CAR-T, showcases a cost-disruptive model. Hospitals require affordable closed systems, in-country QC, and vendor platforms that allow efficient tech transfer. This makes India a value-driven market for enabling technologies.
Thailand
Thailand’s conditional approval framework for ATMPs signals regulatory openness. Vendors offering decentralized manufacturing tools and pharmacovigilance-friendly platforms will find traction.
Taiwan
Taiwan offers first-in-human approvals in as little as 30 days and has deep CAR-T experience. This is an ideal environment for early evaluation of compact processing systems, integrated data packages, and analytics platforms.
Middle East
Israel
Israel is a powerhouse for CGT startups and rapid clinical iteration. Compact bioprocess hardware, digital analytics, and novel delivery tools can find quick uptake and serve as proving grounds for global scale.
United Arab Emirates
The UAE’s Abu Dhabi Stem Cells Center (ADSCC) is running trials in cell therapies, while G42 Healthcare invests in AI and advanced clinical research. This signals demand for analytics, automation, and quality systems beyond traditional cleanroom builds. Vendors offering QC labs, turnkey closed systems, and digital solutions are well positioned.
Saudi Arabia
King Faisal Specialist Hospital in Riyadh is leading CAR-T clinical trial development. Vision 2030 prioritises biotech and regenerative medicine, backed by sovereign wealth funding. Demand signals include automated CAR-T expansion platforms, digital QC, and workforce training aligned with rapid scaling ambitions.
Qatar
Sidra Medicine in Doha is developing regenerative medicine programs and rare disease genomics. Government R&D investment in precision medicine is strong, with opportunities for vendors in genome editing tools, sequencing analytics, and rare disease-focused QC solutions.
Africa
Nigeria
Nigeria has the world’s largest sickle cell patient population. With the global approval of gene-editing therapies like CASGEVY®, attention is turning to Nigeria as a market for future access. The challenge is to translate breakthrough therapies into resource-limited health systems. For enabling technology providers, this signals urgent demand for simplified, portable, and low-cost platforms that could make advanced therapies deliverable at scale.
South Africa
South Africa has the continent’s strongest clinical trial infrastructure, with hubs in Johannesburg and Cape Town and facilities linked to Wits and Stellenbosch universities. Opportunities include viral vector analytics, workforce training platforms, and in-process QC tools for hematology and oncology trials.
Egypt
Egypt has invested in stem cell and gene therapy R&D with government support for ATMP clinical frameworks. Partnerships with European bodies are growing. Vendors in process development, QC, and modular cleanrooms can find early adoption pathways.
Europe
United Kingdom
The UK is leading with new frameworks for point-of-care and modular manufacturing. Compact cleanroom pods, digital QMS, and automation tools that integrate directly into hospitals are in high demand.
Ireland
Ireland is scaling through NIBRT’s new ATMP plant and public funding for newborn cell therapy. Vendors in robotics, analytics, and workforce training can connect directly into this ecosystem.
Germany
Germany’s Berlin and Munich clusters are central to European CGT manufacturing. Robotics-ready platforms, viral vector scale-out technologies, and MES solutions are priorities.
France
France is strengthening its hospital-linked GMP clusters in Paris and Lyon. Vendors with QC analytics and compact processing hardware will find strong pull-through here.
Spain
Catalonia and Andalusia are expanding infrastructure and clinical trial capacity. This is creating demand for comparability analytics, digital QC, and trial-ready processing platforms.
Sweden
Sweden’s Karolinska Institutet and Testa Center are key innovation hubs. The country is a launchpad for automation and closed-system process technologies.
Norway
The Oslo Cancer Cluster is becoming a trial hub for CGT. Vendors supporting trial logistics, cold-chain integration, and biobank systems will find an active customer base.
Denmark
Copenhagen’s life science cluster is investing heavily in ATMP readiness. Robotics, automation, and advanced process control tools are a natural fit.
Italy
Italy’s CGT activity is clustered in Milan and Naples. Consortia-driven programs need potency assays, QC analytics, and hospital-integrated manufacturing solutions.
Austria
Austria’s Vienna ecosystem is entering the ATMP race. Modular suites, electronic batch records, and advanced analytics platforms can accelerate uptake.
The Americas
Brazil
Brazil’s CGT field is growing under evolving ANVISA regulation but remains cost-constrained. Vendors who can deliver closed automation and in-process analytics that reduce cost of goods will resonate.
United States
The US remains the global leader with continued ATMP approvals, including pz-cel for RDEB. Scale-up and scale-out platforms, potency assays, and chain-of-identity systems are now essential infrastructure.
Canada
Canada is combining OmniaBio and CCRM with federal investment in AI and robotics. This creates demand for automation, MES integration, and QC technologies that support advanced manufacturing.
Closing Reflections
Our world tour underscores that CGT is global, yet not uniform. Each region presents its own opportunities and challenges, and for tools and enabling technology providers the lesson is clear: align with local drivers, but keep a global perspective.
At Lonrú Consulting, we specialize in helping enabling technology companies identify and act on these opportunities. Our VantagePoint™ suite brings together market intelligence, validation frameworks, and positioning strategies to ensure technologies not only launch but scale successfully in the dynamic CGT landscape.
The Business of Reaching Patients: ESGCT 2025 Preview
2025’s most important cell and gene therapy (CGT) conversations focus on how we pay, where we manufacture, and who gets access to therapies. ESGCT’s programme quietly concentrates several talks that sit right at that junction. Below are the sessions we’re watching for signals on real-world access, sustainable economics, and the operating models that will define CGT’s next chapter.
1. Clinical Trials & Commercialisation Workshop
(Tuesday, a.m.)
Theme: pricing, access, and launch realism.
Expect a candid sweep from regulatory mechanics to payer expectations and patient‑group perspectives. Two standouts:
Reimbursement models for ATMPs: a practical tour of contracts (outcome‑based, annuity, hybrid) and the data demands they impose on sponsors.
Advanced therapies in rare disease: research to commercial developer‑side lessons on first approvals, launch phasing, manufacturing readiness and payer evidence.
What to listen for: Where multi‑year outcomes contracts are actually landing; how to “design for reimbursement” early (endpoints, real-world-evidence capture, and post‑market commitments built into the plan).
2. In Vivo Gene Therapy
(Wednesday, 15:30–17:30)
This block is a litmus test for one‑and‑done value narratives. The way durability, safety, and quality of life are framed here will influence payer appetite and trial design across the board, especially for programs signaling pivotal readiness. Watch for language around post‑approval data, registry infrastructure, and the end of lifelong chronic therapy in specific conditions.
3. Manufacturing I: Delivery Technologies
(Wednesday, 18:00–19:30)
Session highlight: INV30 A Strategic Platform Roadmap for Commercializing Gene Therapy - Cesar Trigueros (Viralgen).
This is likely to read as an operating model talk with a view to CMC: platform efficiencies, indication sequencing, COGS sensitivity, QC and comparability, and what “commercial‑ready” actually looks like for vector manufacturing in 2025.
Also worth catching in the same session: practical levers that move the unit economics viral vector cell‑line engineering, downstream simplification, and analytical automation, for sponsors who need cost curves that stand up to HTA scrutiny.
4. Accessibility of Gene Therapy
(Thursday, 15:30–17:30)
A rare, concentrated discussion of how to reach more patients: logistics, site qualification, patient access, and payer readiness. Expect pragmatic perspectives from hospital systems and regulators on topics like:
Shipment, chain‑of‑identity, and turn‑around constraints that still block scale.
What “fair access” and “practical affordability” look like for ultra‑rare populations.
How to structure evidence generation so that pricing debates become data‑driven rather than aspirational.
5. Manufacturing II: Cell Therapies
(Thursday, 18:00–19:30)
Session highlight: INV60 Decentralized, innovative point‑of‑care CAR‑T manufacturing platform - Ruiz Astigarraga (Galapagos BV).
This is the business model experiment many have been waiting to see at scale: push manufacturing closer to the bedside to compress vein‑to‑vein, increase throughput, and reduce logistics risks.
Listen for:
What the real time gains are (collection → release).
How digital release, comparability, and QA work across a network of centers.
The site‑level economics (capex/opex, staffing, training) and who pays for what.
Related talks in this block - TIL manufacturing, cryo dynamics, and LVV platform learnings - fill in the operational detail that determines whether “point‑of‑care” is a headline or a workable system.
6. National Initiatives in Gene & Cell Therapy
(Thursday, 18:00–19:30)
This session maps country‑level frameworks for scaling academic manufacturing, coordinated trials, and patient access and how infrastructure and philanthropy‑backed models may intersect with national health systems to broaden availability.
What to listen for:
How Germany, Spain, Italy, France, the UK and Sweden are organizing capacity and standards.
Whether cross‑border data and release protocols are converging enough to reduce duplication and cost.
The role of hospital exemption and academia‑industry hybrids in earlier patient access.
7) Regulatory Requirements for CTAs
(Friday, 11:00–13:00)
With senior voices from agencies, expect clarity on:
First‑in‑human expectations for manufacturing and control.
How the evolving EU legislative landscape interacts with CGT development timelines.
What regulators want to see in post‑approval evidence to support sustained access and reimbursement.
8) EuroGCT Lunchtime Symposium
(Friday, 13:30–14:30)
Policy and regulatory strategy focused on translation and equitable delivery. Watch for signals on registries, real-world-evidence, and center‑of‑excellence concepts that could become the “plumbing” for outcome‑based payment and cross‑site learning.
Lonrú Clients: What this means for CGT enablers
Design for access, not just efficacy. If decentralized and rapid‑release models gain ground, demand rises for modular cleanrooms, integrated analytics, networked QA, and software that makes batch review and chain‑of‑identity auditable across sites.
Plan the contract before the trial. Build outcomes capture, follow‑up cadence, and real‑world endpoints into protocol design to support annuity or outcomes‑based payments later.
Academia is a serious buyer. National initiatives and philanthropic ecosystems (Italy is a prime example) are creating structured channels for tools, analytics, and training - especially where public infrastructure underwrites early access.
The sessions to circle in your diary
Clinical Trials & Commercialisation Workshop (Tuesday, a.m.) pricing, contracting, and the patient perspective.
In Vivo Gene Therapy Plenary (Wednesday, 15:30–17:30) framing “near‑market” for payers.
Manufacturing I (Wednesday, 18:00–19:30) INV30 Cesar Trigueros (Viralgen) on a commercialization roadmap.
Accessibility of Gene Therapy (Thursday, 15:30–17:30) practical access and logistics.
Manufacturing II (Thursday, 18:00–19:30) INV60 Ruiz Astigarraga (Galapagos BV) on decentralized CAR‑T.
National Initiatives (Thursday, 18:00–19:30) include Italy alongside Germany, Spain, France, the UK, Sweden.
Regulatory Requirements for CTAs (Friday, 11:00–13:00) policy reality check.
How Lonrú can help
Lonrú’s VantagePoint™ services support companies turning these signals into action:
Insights: market and policy intelligence to anticipate the access path.
Validation & Accelerate: go‑to‑market design, including networked manufacturing and payer‑ready evidence plans.
Connect & Scale: partnerships with national initiatives, academic centers and investors; pricing and scale frameworks aligned to real‑world delivery.
CRISPR Safety: Where Tools Companies Can Lead
The recent Genes & Diseases review by the CGT Catapult’s team on non-clinical safety considerations for CRISPR/Cas genome editing is a valuable reference for anyone in the enabling technology space. It sets out the practical safety and regulatory challenges that stand between promising science and first-in-human use. For companies developing tools and platforms, it offers a clear view of where targeted solutions can become part of the industry’s essential infrastructure.
At Lonrú Consulting, we work with CGT-enabling technology providers to identify exactly these kinds of opportunities. Using our VantagePoint™ framework, we help clients align product development with market demand, validate their technology for partner and regulator confidence, and plan for commercial growth. In the sections that follow, we examine the primary safety concerns highlighted in the recent CRISPR/Cas review, explore the corresponding opportunities for tools companies, and indicate where our VantagePoint™ solutions can support those companies in advancing their mission.
The recent review by Toofan et al. outlines six key safety considerations for CRISPR/Cas editing, we outline opportunities for tools companies to address each point.
1. Off-Target and On-Target-But-Unintended Edits
A starting point for many developers is the challenge of controlling where and how genome edits occur. The review details the potential for unwanted changes both at and away from the target site, ranging from single base substitutions to megabase-scale deletions and rearrangements. Regulatory bodies increasingly expect comprehensive datasets that quantify these effects and show how they are being mitigated.
Opportunity for tools companies: Develop AI-assisted gRNA design systems that take into account chromatin state and sequence context, engineer high-fidelity Cas variants, and provide GLP-ready detection platforms such as NGS, ddPCR, and single-cell sequencing.
VantagePoint™ Fit: Insights to analyze adoption rates and emerging technical standards, and Validation to format and present results in a way that satisfies regulatory expectations.
2. p53-Mediated Toxicity and Tumorigenicity
The second major concern is the unintended cellular response to double-strand breaks. The review describes how activation of p53 pathways can result in apoptosis, or in some cases, selective survival of p53-deficient cells with a higher likelihood of tumorigenicity. This risk profile can vary by target site and cell type, making it a key focus for preclinical safety assessment.
Opportunity: Create assay kits to measure p53 activity in edited cells, develop DSB-free editing technologies such as base or prime editors with companion validation tools, and design genomic stability assays for iPSC-derived and primary cell therapies.
VantagePoint™ Fit: Accelerate by guiding high-value applications for DSB-free platforms, and Illuminate by helping you communicate the safety value of your solution to potential partners and regulators.
3. Immunogenicity to Cas Proteins and Delivery Vectors
Another recurring issue in the paper is the immune system’s response to both the editing protein and the delivery vehicle. Many individuals already carry antibodies or T cells reactive to Cas proteins, and delivery methods such as AAV and LNP can trigger strong innate or adaptive immune responses. These reactions can undermine efficacy and limit repeat dosing.
Opportunity: Engineer Cas variants with reduced immune recognition, identify alternative orthologs from under-exposed bacterial species, and develop immune-profiling services to assess patient compatibility with specific delivery systems.
VantagePoint™ Fit: Connect by linking your technology to immune assay providers and therapy developers, and Scale by embedding immune risk management into development pipelines that aim for repeat administration.
4. Delivery System Limitations
The choice of delivery method shapes both the feasibility and safety of a genome editing approach. AAV vectors face strict size limits and potential high-dose toxicities, adenovirus offers larger capacity but carries a higher risk of immune activation, and LNPs can be unstable or inefficient for certain tissue types. The review notes that efficient, targeted delivery remains one of the biggest barriers to translation.
Opportunity: Create modular delivery systems that overcome payload constraints, develop hybrid viral/non-viral approaches, and offer AI-driven biodistribution modeling for early-stage planning.
VantagePoint™ Fit: Insights to track market shifts in delivery technology adoption, and Validation to provide preclinical data packages that can support licensing or partnership agreements.
5. Bio-distribution, Persistence, and Clearance
Where the editing system goes in the body, how long it remains active, and whether it lingers in non-target tissues are all central to non-clinical risk assessment. These parameters can change significantly depending on vector type, dosing, and administration route. For cell-based products, especially allogeneic or iPSC-derived, long-term tracking is a critical requirement.
Opportunity: Develop tracking technologies that are applicable from preclinical to clinical settings, create predictive PK/PD modelling platforms, and provide integration site mapping services.
VantagePoint™ Fit: Scale by positioning your product as a standard solution for generating long-term safety data, and Accelerate by enabling developers to present robust bio-distribution findings early in regulatory interactions.
6. Regulatory-Grade Non-Clinical Packages
Finally, the review emphasizes the need for non-clinical data packages that are tailored to the product, indication, and intended patient population. Both FDA and EMA stress a risk-based approach, with GLP compliance where relevant, and a focus on answering the most critical safety questions for each use case. Delivering this efficiently can shorten timelines and reduce cost.
Opportunity: Offer ready-to-use regulatory data generation templates, integrate validated assay panels with in silico and in vitro modeling, and create audit-ready dashboards for submissions.
VantagePoint™ Fit: Illuminate your technology as a tool for faster regulatory readiness, and Validation to show how your offering aligns with agency requirements without adding unnecessary study burden.
Final note:
The safety challenges discussed in the review are defining factors in whether a therapy advances to the clinic. For enabling technology companies, each represents a space where a well-designed tool or platform can become essential to the development process. The VantagePoint™ approach gives you a way to identify these openings, build the case for your solution, connect with the right partners, and create a clear path from innovation to market impact.
A global view on point of care manufacturing regulations after the UK’s leap
The UK’s Human Medicines (Amendment) (Modular Manufacture and Point of Care) Regulations 2025 discussed in last week’s post, flipped the regulatory lens from post-code to process. By creating two new licenses; one for fixed hospital suites and one for relocatable micro-factories, with a single control site supervising every node through a live quality-management master file, the MHRA has written the world’s first clear pathway for turning cell, gene and other ultra-short-shelf-life products from a centralized craft into a distributed service.
For developers, that means the last, failure-prone hours of a CAR-T or CRISPR-edited product can now happen meters from the patient instead of hundreds of miles away. It also means regulators will review data-integrity safeguards, remote batch-release protocols and digital twins with the same rigor they once reserved for stainless-steel reactors which raises the bar overall, but shortens the critical vein-to-vein time. In the sections that follow we set the UK’s new framework beside emerging approaches in the United States, Japan and China to see which markets are closest to matching the MHRA’s lead and to identify where gaps remain.
Bringing the factory to the patient: we’re tracking the rules that make it possible.
United States: a framework taking shape
Across the Atlantic the FDA is edging toward a similar destination through its CDER FRAME initiative. Since 2022 the Agency has floated discussion papers on Distributed Manufacturing and Point-of-Care Manufacturing of Drugs, held dedicated workshops and, in November 2023, published a stakeholder action plan. The timetable now includes three draft guidances, the first of which (addressing control-strategy verification under 21 CFR 211) landed in January 2025. The concept mirrors the UK model (central QMS, remotely located units) but, for now, manufacturers must patch together existing CGMP provisions and request case-by-case advice while FDA finalizes its rule book.
Japan: a decade of hospital manufacture, newly widened
Japan actually legalised bedside manufacture back in 2014. Under the Act on the Safety of Regenerative Medicine (ASRM) physicians can prepare autologous cells in registered in-hospital suites, regulated site by site, while the Pharmaceuticals and Medical Devices Act (PMD Act) governs commercial products. That twin-track system is now tightening: an ASRM amendment coming into force on 31 May 2025 pulls in vivo gene therapy into the highest-risk tier and demands stronger conflict-of-interest controls and data oversight by Certified Committees for Regenerative Medicine. Japan therefore offers a functioning clinical pathway without mandating the real-time, multi-site master-file structure that the MHRA requires.
China: a maturing dual-track experiment
China’s regulators split advanced-therapy oversight between the National Health Commission (NHC), which lets accredited hospitals run investigator-initiated cell-therapy trials, and the National Medical Products Administration (NMPA), which handles industry INDs and licences. Draft NHC rules piloted in 2021 were finalized in September 2024, standardizing digital supervision and ethics governance for hospital-run trials, while the NMPA continues to refine 2017 and 2021 technical guidelines for gene-modified cells . The model already enables bedside manufacture, but licenses remain site-specific and provincial, and there is no single, central control-site concept, leaving data harmonization and nationwide scalability as open questions.
What this signals
Taken together, these moves show convergence on a core idea: personalized therapies can be finished where the patient is, yet controlled as if they were still made in one building. The UK supplies the first full legal scaffold; the FDA is drafting its solution, Japan is extending an established hospital channel into gene therapy, and China is aligning its fast-growing hospital track with national standards.
For therapeutic innovators the lesson is clear: design digital-first quality systems that can stream data from dozens of small reactors, bake remote intervention into CMC plans, and pick an early pilot jurisdiction in London, Boston or Tokyo to prove the model.
For technology innovators, the engineers building closed, automated cell-processing suites, rapid sterility assays, inline analytics, electronic batch-record platforms and AI-powered deviation handling, the opportunity is just as immediate. Hospitals will invest in turnkey, compliance-by-design micro-factories that drop into existing real estate, streaming validated data back to a control site and that can be overseen, paused or released by a Qualified Person half a continent away. Vendors that can wrap hardware and software into a tamper-proof, cloud-native ecosystem, complete with digital twins, secure SOPs and encrypted audit trails will find the UK an unrivaled proving ground and a springboard for the FDA’s forthcoming guidance.