CRISPR Safety: Where Tools Companies Can Lead
The recent Genes & Diseases review by the CGT Catapult’s team on non-clinical safety considerations for CRISPR/Cas genome editing is a valuable reference for anyone in the enabling technology space. It sets out the practical safety and regulatory challenges that stand between promising science and first-in-human use. For companies developing tools and platforms, it offers a clear view of where targeted solutions can become part of the industry’s essential infrastructure.
At Lonrú Consulting, we work with CGT-enabling technology providers to identify exactly these kinds of opportunities. Using our VantagePoint™ framework, we help clients align product development with market demand, validate their technology for partner and regulator confidence, and plan for commercial growth. In the sections that follow, we examine the primary safety concerns highlighted in the recent CRISPR/Cas review, explore the corresponding opportunities for tools companies, and indicate where our VantagePoint™ solutions can support those companies in advancing their mission.
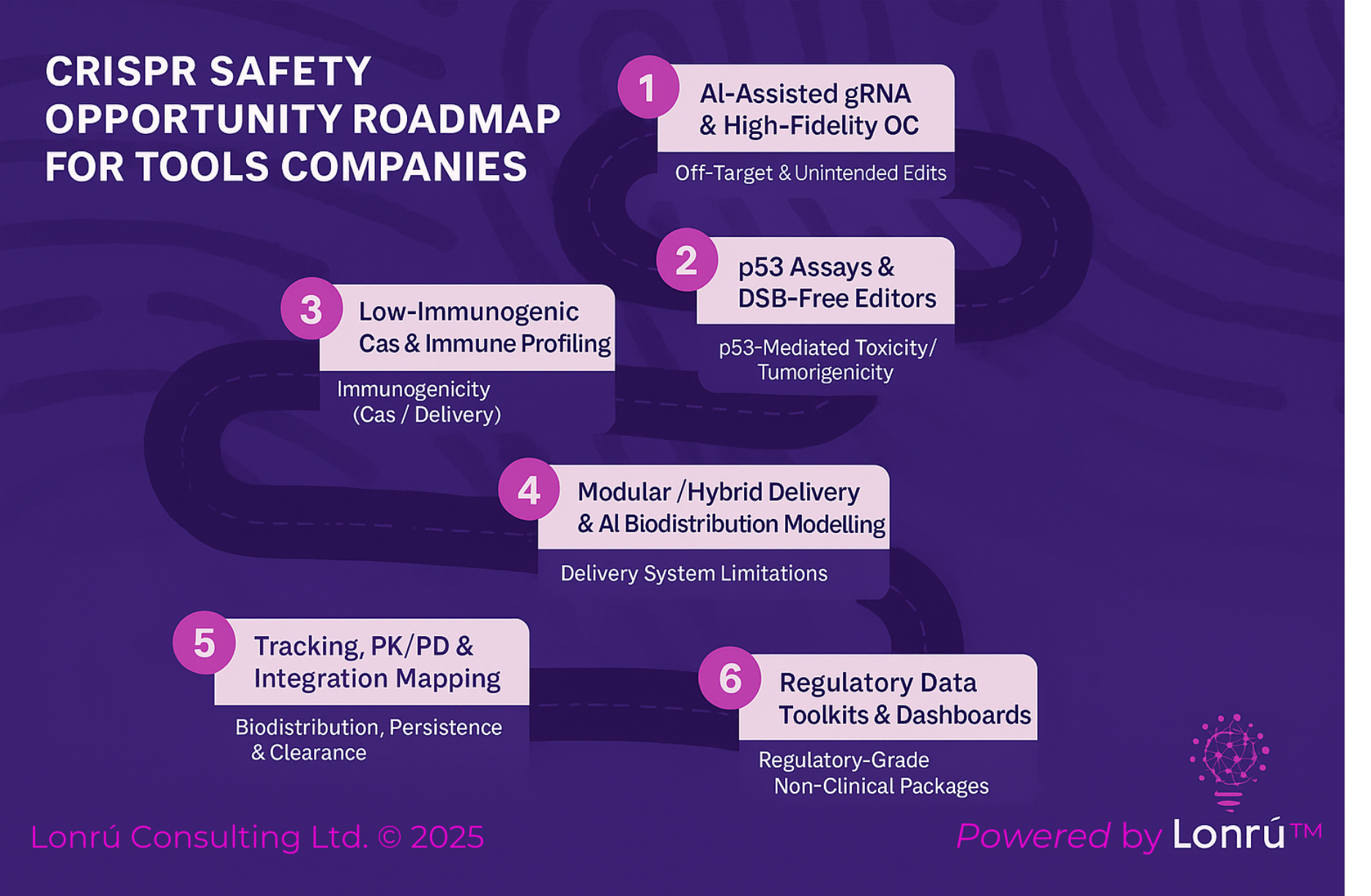
The recent review by Toofan et al. outlines six key safety considerations for CRISPR/Cas editing, we outline opportunities for tools companies to address each point.
1. Off-Target and On-Target-But-Unintended Edits
A starting point for many developers is the challenge of controlling where and how genome edits occur. The review details the potential for unwanted changes both at and away from the target site, ranging from single base substitutions to megabase-scale deletions and rearrangements. Regulatory bodies increasingly expect comprehensive datasets that quantify these effects and show how they are being mitigated.
Opportunity for tools companies: Develop AI-assisted gRNA design systems that take into account chromatin state and sequence context, engineer high-fidelity Cas variants, and provide GLP-ready detection platforms such as NGS, ddPCR, and single-cell sequencing.
VantagePoint™ Fit: Insights to analyze adoption rates and emerging technical standards, and Validation to format and present results in a way that satisfies regulatory expectations.
2. p53-Mediated Toxicity and Tumorigenicity
The second major concern is the unintended cellular response to double-strand breaks. The review describes how activation of p53 pathways can result in apoptosis, or in some cases, selective survival of p53-deficient cells with a higher likelihood of tumorigenicity. This risk profile can vary by target site and cell type, making it a key focus for preclinical safety assessment.
Opportunity: Create assay kits to measure p53 activity in edited cells, develop DSB-free editing technologies such as base or prime editors with companion validation tools, and design genomic stability assays for iPSC-derived and primary cell therapies.
VantagePoint™ Fit: Accelerate by guiding high-value applications for DSB-free platforms, and Illuminate by helping you communicate the safety value of your solution to potential partners and regulators.
3. Immunogenicity to Cas Proteins and Delivery Vectors
Another recurring issue in the paper is the immune system’s response to both the editing protein and the delivery vehicle. Many individuals already carry antibodies or T cells reactive to Cas proteins, and delivery methods such as AAV and LNP can trigger strong innate or adaptive immune responses. These reactions can undermine efficacy and limit repeat dosing.
Opportunity: Engineer Cas variants with reduced immune recognition, identify alternative orthologs from under-exposed bacterial species, and develop immune-profiling services to assess patient compatibility with specific delivery systems.
VantagePoint™ Fit: Connect by linking your technology to immune assay providers and therapy developers, and Scale by embedding immune risk management into development pipelines that aim for repeat administration.
4. Delivery System Limitations
The choice of delivery method shapes both the feasibility and safety of a genome editing approach. AAV vectors face strict size limits and potential high-dose toxicities, adenovirus offers larger capacity but carries a higher risk of immune activation, and LNPs can be unstable or inefficient for certain tissue types. The review notes that efficient, targeted delivery remains one of the biggest barriers to translation.
Opportunity: Create modular delivery systems that overcome payload constraints, develop hybrid viral/non-viral approaches, and offer AI-driven biodistribution modeling for early-stage planning.
VantagePoint™ Fit: Insights to track market shifts in delivery technology adoption, and Validation to provide preclinical data packages that can support licensing or partnership agreements.
5. Bio-distribution, Persistence, and Clearance
Where the editing system goes in the body, how long it remains active, and whether it lingers in non-target tissues are all central to non-clinical risk assessment. These parameters can change significantly depending on vector type, dosing, and administration route. For cell-based products, especially allogeneic or iPSC-derived, long-term tracking is a critical requirement.
Opportunity: Develop tracking technologies that are applicable from preclinical to clinical settings, create predictive PK/PD modelling platforms, and provide integration site mapping services.
VantagePoint™ Fit: Scale by positioning your product as a standard solution for generating long-term safety data, and Accelerate by enabling developers to present robust bio-distribution findings early in regulatory interactions.
6. Regulatory-Grade Non-Clinical Packages
Finally, the review emphasizes the need for non-clinical data packages that are tailored to the product, indication, and intended patient population. Both FDA and EMA stress a risk-based approach, with GLP compliance where relevant, and a focus on answering the most critical safety questions for each use case. Delivering this efficiently can shorten timelines and reduce cost.
Opportunity: Offer ready-to-use regulatory data generation templates, integrate validated assay panels with in silico and in vitro modeling, and create audit-ready dashboards for submissions.
VantagePoint™ Fit: Illuminate your technology as a tool for faster regulatory readiness, and Validation to show how your offering aligns with agency requirements without adding unnecessary study burden.
Final note:
The safety challenges discussed in the review are defining factors in whether a therapy advances to the clinic. For enabling technology companies, each represents a space where a well-designed tool or platform can become essential to the development process. The VantagePoint™ approach gives you a way to identify these openings, build the case for your solution, connect with the right partners, and create a clear path from innovation to market impact.