Who Follows Verve?
Cash Runway, Indication “White-Space”, and Where the Tools Ecosystem Can Play A Part
Lilly-Verve: A Wake-Up Call for the Gene-Editing Supply Chain
Eli Lilly’s agreement to acquire Verve Therapeutics for $1.3 billion delivered the first big-pharma endorsement of an in-vivo base-editing therapy aimed at a common cardiovascular risk factor. For developers this affirms that a well-differentiated programme can command a premium while still in early clinical stages; for the companies that supply lipids, vectors, analytical assays, single-cell QC or GMP processes the message is equally clear: the strategic value of an editing platform now rises or falls with the maturity of its tool-chain. In other words, big-pharma is prepared to pay early only when it can see a credible route to scale-up, and that route is paved by the specialist providers working behind the scenes.
Who We’re Tracking, and Why a Few Familiar Names Don’t Appear
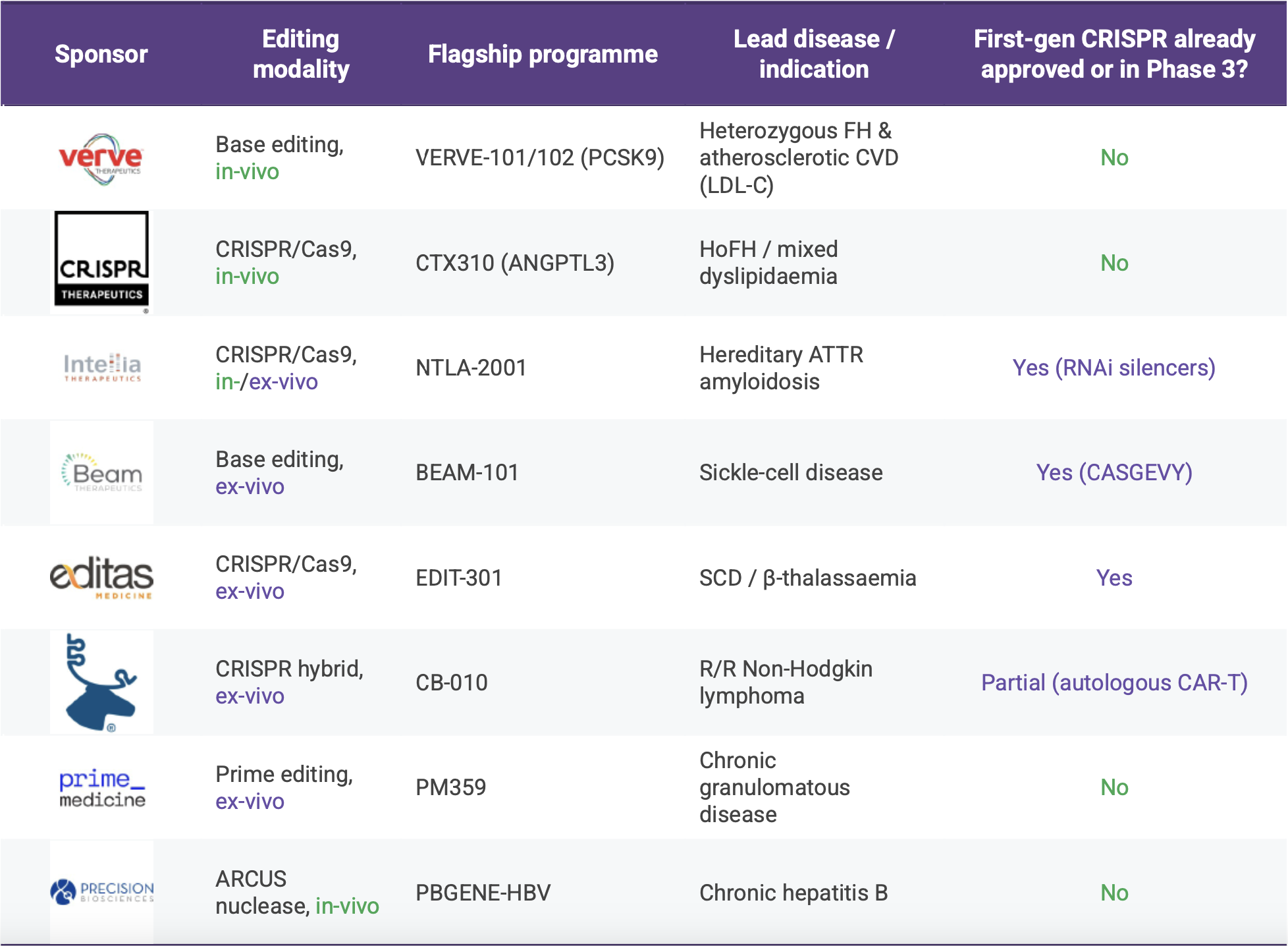
Our lens concentrates on Beam Therapeutics, Intellia Therapeutics, CRISPR Therapeutics, Editas Medicine, Caribou Biosciences, Prime Medicine and Precision BioSciences. Each of these seven companies is publicly listed, still independent, and already has at least one drug-editing programme in the clinic or cleared for first-in-human testing. Recently purchased firms such as Capstan (now inside AbbVie), EsoBiotec (acquired by AstraZeneca) and Poseida (absorbed by Roche) no longer control their own financing cadence, while delivery-only or RNA-editing specialists like ReCode, ShapeTX and Korro tend to measure progress by platform milestones rather than therapeutic read-outs. Note, Precision’s ARCUS technology earns its place even though it is derived from a homing endonuclease rather than Cas9: it performs the same sequence-specific cut in an in-vivo setting and therefore competes for exactly the same opportunities.
White-Space Shots vs. Second-Wave Plays: The Current Indication Map
Within that group the spectrum of ambition is striking. Verve, now partnered with Lilly, and CRISPR Therapeutics are pushing into cardiovascular lipid disorders where no first-generation genome editor has yet secured approval, while Intellia is offering a one-and-done alternative to the chronic RNA-interference treatments already in play for ATTR amyloidosis. Intellia also reported fresh NTLA-2002 data yesterday, boosting investor sentiment but not altering its runway guidance. Programmes from Beam, Editas and Caribou focus on haemoglobinopathies or oncology indications that regulators have already seen in an autologous or first-wave CRISPR guise, making differentiation a question of efficiency, safety and patient access. Prime Medicine and Precision BioSciences step into ultra-rare chronic granulomatous disease and chronic hepatitis B respectively, both areas without an existing curative option. We have added Verve/Lilly to the indication table so we can compare how the acquired asset fits alongside the still-independent cohort.
Source: Lonrú Consulting’s VantagePoint™ analysis, July 2025. The post-Verve field of clinical-stage gene-editors and where each sits on the white space v second wave spectrum.
Charting runway next to market size, and why Verve serves as a useful benchmark
Mapping each company’s publicly guided cash runway on one axis and the projected 2030 market size of its lead indication on the other helps reveal who may seek partnership cash soonest and who can afford to take a longer view on tool selection. The Verve/Lilly transaction provides a helpful reference point: Verve entered the deal with roughly twenty-four months of runway remaining and was addressing a market comfortably north of twenty-five billion dollars. Any company that shows a shorter runway or a smaller commercial opportunity may need to demonstrate an even tighter command of its supply chain, or else invite partnership earlier than planned. For tool providers this plot highlights two complementary paths: larger-TAM, longer-runway players can commit to multi-year, higher-margin supply agreements, whereas shorter-runway firms may look for creative cost-sharing structures that still ensure uninterrupted development.
Source: Lonrú Consulting’s VantagePoint™ analysis (July 2025). Cash-runway figures drawn from latest SEC filings; 2030 market sizes from consensus analyst and disease-area forecasts.
From the supplier’s perspective the priorities differ by modality. In-vivo cardio and hepatitis programmes rely on ever finer lipid chemistry, capsid engineering and genome-wide off-target analytics; vendors that can package those capabilities together become attractive long-term partners. Ex-vivo haem-onc projects remain sensitive to cost of goods and therefore reward vendors who can combine closed-system cell hardware with lean vector manufacture or cost effective alternatives. Throughout, the tone is shifting from transactional reagent sales to collaborative co-development aimed at building IND-ready modules that regulators will recognise.
Signals to Watch and Practical Moves for Lonrú Clients
Regulators are already signalling an interest in longer durability and safety read-outs for single-shot LDL-C and HBV cures, a change that is likely to boost demand for high-resolution sequencing assays and longitudinal biomarker platforms. Macroeconomic uncertainty means companies holding less than eighteen months of cash could accelerate partnering or M&A conversations, potentially redrawing the competitive field in a matter of quarters. Meanwhile, any early clinical success from prime editing or ARCUS could reshuffle licensing appetites in much the same way Verve’s early cardio data did in 2024.
For Lonrú’s clients the immediate takeaway is to align commercial efforts with those scientific and financial inflection points. Cardiometabolic and hepatitis programmes offer the largest revenue head-room and will tolerate premium pricing if the tools accelerate path-to-market, whereas the haem-onc field will reward solutions that deliver efficiency and regulatory clarity at a competitive cost. Engaging earlier; ideally before a cash-short biotech reaches its fund-raising cliff, creates room for more balanced, longer-term collaboration that can survive the inevitable shifts that follow a strategic acquisition.